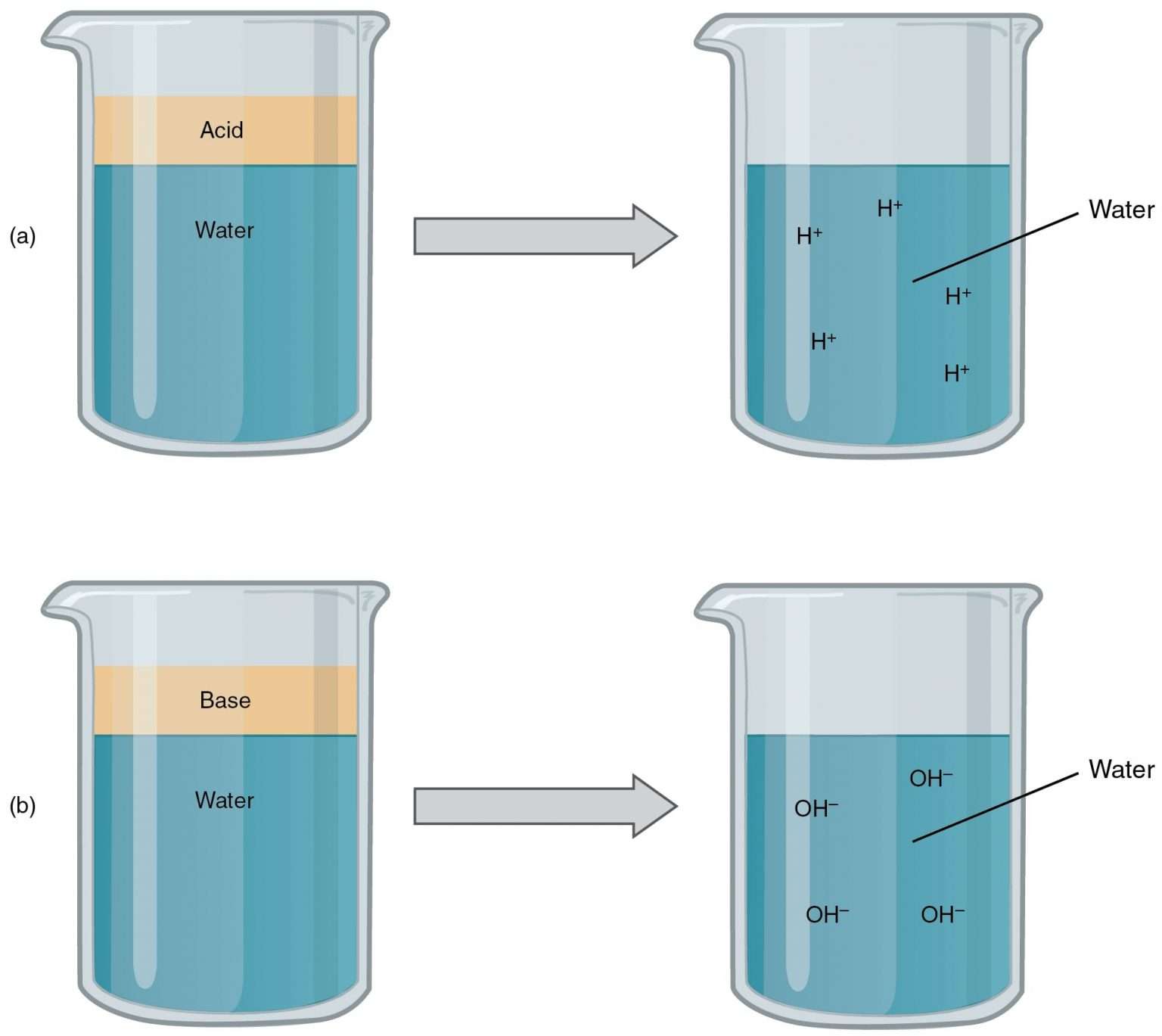
Acids React With Bases To Form
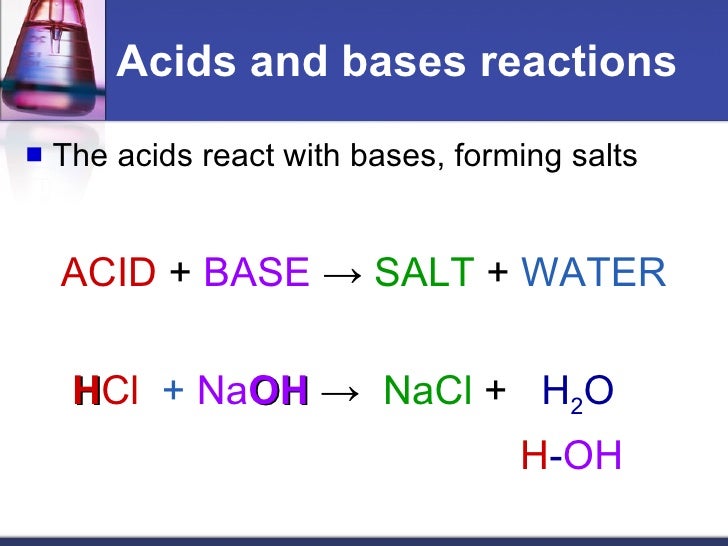
Acids React With Bases To Form - Acids react with bases to form a salt and water. Web acids react with bases to produce a salt compound and water. Web terms in this set (11) acids react with bases to form a ___________ and a ____________. Web acids react with metals, bases and carbonates to produce salts. The neutralization of a strong acid and strong base has a ph equal to 7. Part of chemistry (single science) chemistry of. Acid + base → salt + water. This could be because it is a hydroxide salt, like naoh, or. Web acid reacts with bases to form __________. Neutralisation is the reaction between an acid and a base.
Web a base is an electrolyte (strong or weak) that produces hydroxide ions when dissolved in water solution. Web answer acids and bases react with metals acids react with most metals to form a salt and hydrogen gas. This could be because it is a hydroxide salt, like naoh, or. When zinc (zn) reacts with dilute sulphuric acid. Ammonia is not a strong base. Web in fact, this reaction is so iconic that we define a base as any compound that undergoes neutralisation reaction with acids. Web an acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form. Acid + metals → salt + hydrogen gas. Web acids react with bases to produce a salt compound and water. Web bases react with acids to neutralize each other at a fast rate both in water and in alcohol.
Web terms in this set (11) acids react with bases to form a ___________ and a ____________. Neutralisation is the reaction between an acid and a base. Acids react with bases to form a salt and water. Acids change litmus (a blue vegetable. Web acids react with bases to produce a salt compound and water. Web bases react with acids to neutralize each other at a fast rate both in water and in alcohol. As discussed in chapter 7, metals that are more active. When zinc (zn) reacts with dilute sulphuric acid. A salt b salt + water c salt + o 2 d salt + h 2 hard solution verified by toppr correct option is b) in a neutralisation reaction, an acid. Web when an acid and a base are placed together, they react to neutralize the acid and base properties, producing a salt.
CHAPTER 2 ACIDS BASES SALTS 1 Acknowledgment Images
Web bases react with acids to neutralize each other at a fast rate both in water and in alcohol. Neutralisation is the reaction between an acid and a base. Web answer acids and bases react with metals acids react with most metals to form a salt and hydrogen gas. Web acids react with metals, bases and carbonates to produce salts..
Acids Bases
This could be because it is a hydroxide salt, like naoh, or. Web in fact, this reaction is so iconic that we define a base as any compound that undergoes neutralisation reaction with acids. Web acids react with bases to produce a salt compound and water. Web acid reacts with metals to form salt and hydogen gas is also released..
Properties and Uses of Acids Lesson Plan Coaches
When zinc (zn) reacts with dilute sulphuric acid. An ionic compound formed from the positive ion of the. A salt b salt + water c salt + o 2 d salt + h 2 hard solution verified by toppr correct option is b) in a neutralisation reaction, an acid. Web acid reacts with bases to form __________. Ammonia is not.
Acids, bases and salts CPD RSC Education
Neutralisation is the reaction between an acid and a base. Web acid reacts with bases to form __________. Web in fact, this reaction is so iconic that we define a base as any compound that undergoes neutralisation reaction with acids. When dissolved in water, the strong base sodium hydroxide ionizes into hydroxide and. Web solution a neutralization reaction is when.
Lecture 19.1a Acid/Base Properties
An ionic compound formed from the positive ion of the. Acid + base → salt + water. Web acids react with metals, bases and carbonates to produce salts. Acids change litmus (a blue vegetable. Web bases react with acids to neutralize each other at a fast rate both in water and in alcohol.
AcidBase Extraction Chemistry LibreTexts
Web acids react with bases to produce a salt compound and water. The neutralization of a strong acid and strong base has a ph equal to 7. Web acid, any substance that in water solution tastes sour, changes the colour of certain indicators (e.g., reddens blue litmus paper), reacts with some metals (e.g., iron) to. This could be because it.
The Chemistry of Acids and Bases Presentation Chemistry
Web solution a neutralization reaction is when an acid and a base react to form water and a salt. A salt b salt + water c salt + o 2 d salt + h 2 hard solution verified by toppr correct option is b) in a neutralisation reaction, an acid. Acid + metals → salt + hydrogen gas. When zinc.
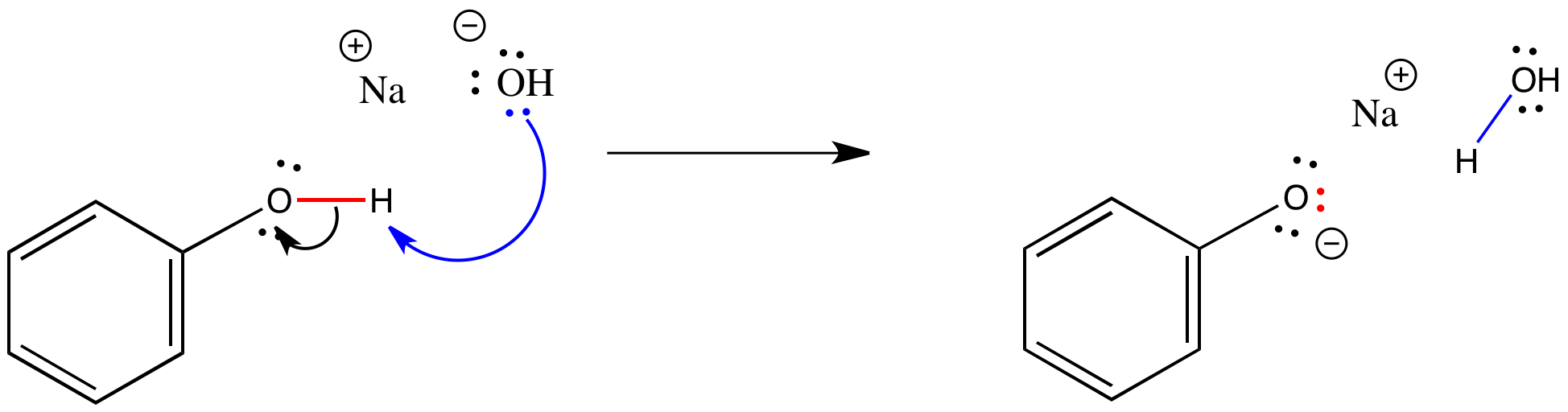
Solved Carboxylic acids react with bases such as NaOH to
Web acids react with bases to produce a salt compound and water. It is a strong acid. Acids change litmus (a blue vegetable. Web acid reacts with bases to form __________. A salt b salt + water c salt + o 2 d salt + h 2 hard solution verified by toppr correct option is b) in a neutralisation reaction,.
How do acids and bases react with each other Neutralization Reaction
When dissolved in water, the strong base sodium hydroxide ionizes into hydroxide and. Web bases react with acids to neutralize each other at a fast rate both in water and in alcohol. Taste sour (don't taste them!)—the word 'acid' comes from the latin acere, which means 'sour'. Acids react with bases to form a salt and water. Web terms in.
Acids and Bases 9 Properties, Useful Reaction & Examples
The neutralization of a strong acid and strong base has a ph equal to 7. Web an acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form. Acids change litmus (a blue vegetable. Web answer acids and bases react with.
As Discussed In Chapter 7, Metals That Are More Active.
Web bases react with acids to neutralize each other at a fast rate both in water and in alcohol. Web when an acid and a base are placed together, they react to neutralize the acid and base properties, producing a salt. When dissolved in water, the strong base sodium hydroxide ionizes into hydroxide and. Web terms in this set (11) acids react with bases to form a ___________ and a ____________.
Acid + Base → Salt + Water.
Neutralisation is the reaction between an acid and a base. Web a base is an electrolyte (strong or weak) that produces hydroxide ions when dissolved in water solution. An ionic compound formed from the positive ion of the. Part of chemistry (single science) chemistry of.
It Is A Strong Acid.
When zinc (zn) reacts with dilute sulphuric acid. Web answer acids and bases react with metals acids react with most metals to form a salt and hydrogen gas. Web an acid in a water solution tastes sour, changes the colour of blue litmus paper to red, reacts with some metals (e.g., iron) to liberate hydrogen, reacts with bases to form. Web acids react with bases to produce a salt compound and water.
Web Acid, Any Substance That In Water Solution Tastes Sour, Changes The Colour Of Certain Indicators (E.g., Reddens Blue Litmus Paper), Reacts With Some Metals (E.g., Iron) To.
Web in fact, this reaction is so iconic that we define a base as any compound that undergoes neutralisation reaction with acids. Web solution a neutralization reaction is when an acid and a base react to form water and a salt. Ammonia is not a strong base. The neutralization of a strong acid and strong base has a ph equal to 7.
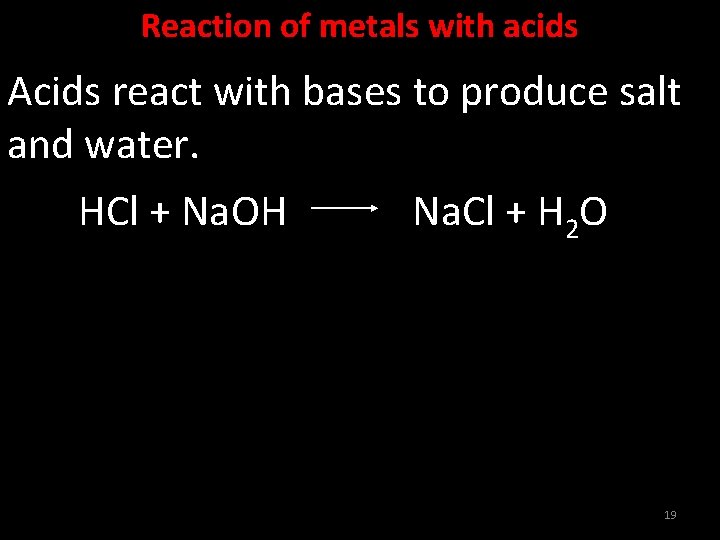


.PNG)