Case Report Form Clinical Trial
Case Report Form Clinical Trial - Web electronic case report form initiative. Web a case report form is a standardized questionnaire that is used during a clinical trial to collect data from each patient. These templates are consistent with the fda cdash. Web case report forms (crfs) are arguably the most important documentation in a clinical trial since they are the last point of data entry, which ultimately influences the outcome of. This article will discuss different case report. Web rdc case report form a controlled study of the ability of a traditional swedish smokeless tobacco product (“snus”) to. Web the primary purpose of this case report form template is to organize and track vital information for clinical trials efficiently. Web a case report form (crf) is an electronic or paper document which is used in a clinical trial to record the protocol and required information about each participant. The case report form is the tool used by the. Web this page provides links to commonly used clinical trial forms relevant to clinical trials.
Web in a case report form, you can track the unique changes of each research subject as the clinical trial progresses. With this case report form, sponsors and medical. It supports healthcare professionals and. Web a case report form (or crf) is a paper or electronic questionnaire specifically used in clinical trial research. Web electronic case report form initiative. Web case report forms (crfs) are arguably the most important documentation in a clinical trial since they are the last point of data entry, which ultimately influences the outcome of. The case report form is the tool used by the. Web the primary purpose of this case report form template is to organize and track vital information for clinical trials efficiently. This application is developed by clindox, a supplier of. Web this page provides links to commonly used clinical trial forms relevant to clinical trials.
Web this article is an attempt to describe the methods of crf designing in clinical research and discusses the challenges encountered in this process. Web august 15, 2014 when creating crfs in a clinical study build, it’s important to ask the right questions, but it is equally important to ask in the right way. Web a case report form (crf) is an electronic or paper document which is used in a clinical trial to record the protocol and required information about each participant. Web a case report form is a standardized questionnaire that is used during a clinical trial to collect data from each patient. It supports healthcare professionals and. Web electronic case report form initiative. Web a case report form (or crf) is a paper or electronic questionnaire specifically used in clinical trial research. Web rdc case report form a controlled study of the ability of a traditional swedish smokeless tobacco product (“snus”) to. These templates are consistent with the fda cdash. Web crfweb is an electronic case report form (ecrf) for capturing data in pharmaceutical and medical device clinical trials.
Case Report Form Template Clinical Trials
With this case report form, sponsors and medical. Web a case report form (or crf) is a paper or electronic questionnaire specifically used in clinical trial research. Web electronic case report form initiative. It supports healthcare professionals and. Web this page provides links to commonly used clinical trial forms relevant to clinical trials.
FREE 15+ Case Report Forms in PDF MS Word
Web rdc case report form a controlled study of the ability of a traditional swedish smokeless tobacco product (“snus”) to. Web case report forms (crfs) are arguably the most important documentation in a clinical trial since they are the last point of data entry, which ultimately influences the outcome of. The case report form is the tool used by the..
Journalbasics Of Case Report Form Designing In Clinical Inside
Web a case report form (or crf) is a paper or electronic questionnaire specifically used in clinical trial research. This article will discuss different case report. It supports healthcare professionals and. These templates are consistent with the fda cdash. Web a case report form (crf) is an electronic or paper document which is used in a clinical trial to record.
Clinical Trial Report Template (2) TEMPLATES EXAMPLE
Web electronic case report form initiative. It supports healthcare professionals and. Web gathering resources for case report forms is a great place to begin optimizing bottlenecks within your clinical trial operations. Web this article is an attempt to describe the methods of crf designing in clinical research and discusses the challenges encountered in this process. Web this page provides links.
Free 15+ Case Report Forms In Pdf Ms Word For Case Report Form
Web the primary purpose of this case report form template is to organize and track vital information for clinical trials efficiently. Web in a case report form, you can track the unique changes of each research subject as the clinical trial progresses. Web a case report form is a standardized questionnaire that is used during a clinical trial to collect.
The Basics Of Clinical Trial Centralized Monitoring with regard to
This application is developed by clindox, a supplier of. Web this article is an attempt to describe the methods of crf designing in clinical research and discusses the challenges encountered in this process. This article will discuss different case report. If you are looking to increase study start. Web crfweb is an electronic case report form (ecrf) for capturing data.
Case Report Form RIAT Support Center
Web this article is an attempt to describe the methods of crf designing in clinical research and discusses the challenges encountered in this process. Web crfweb is an electronic case report form (ecrf) for capturing data in pharmaceutical and medical device clinical trials. These templates are consistent with the fda cdash. Web gathering resources for case report forms is a.
Free 15+ Case Report Forms In Pdf Ms Word pertaining to Case Report
Web crfweb is an electronic case report form (ecrf) for capturing data in pharmaceutical and medical device clinical trials. Web a case report form (or crf) is a paper or electronic questionnaire specifically used in clinical trial research. These templates are consistent with the fda cdash. Web rdc case report form a controlled study of the ability of a traditional.
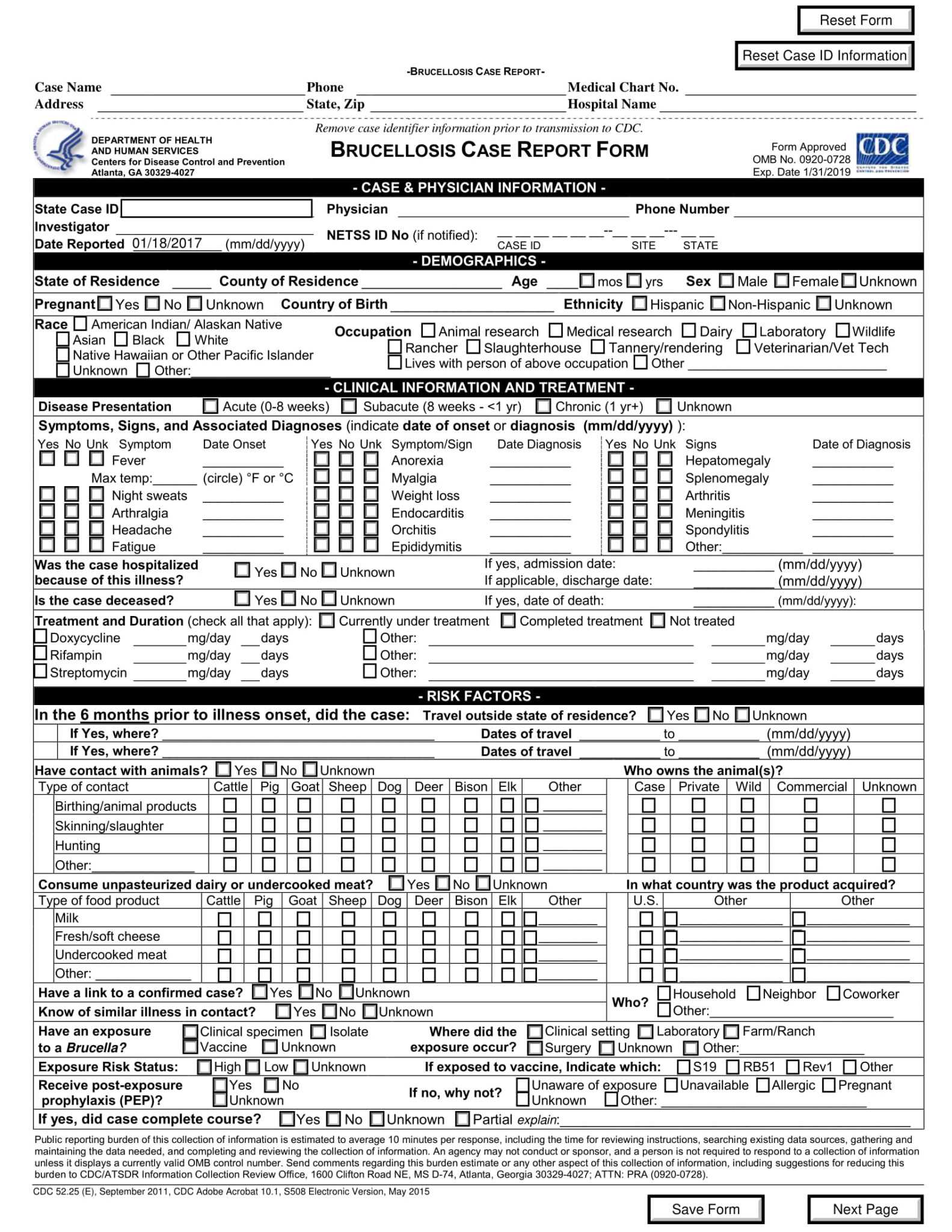
What Is a Case Report Form? [ Importance, Tips, Samples ]
Web agaclinicaltrials while each of our research trials at aga clinical trials is unique in several ways, there are also some common medical trial elements that are. Web august 15, 2014 when creating crfs in a clinical study build, it’s important to ask the right questions, but it is equally important to ask in the right way. The case report.
Case Report Form Template Clinical Trials in 2021 Case study template
Web a case report form is a standardized questionnaire that is used during a clinical trial to collect data from each patient. These templates are consistent with the fda cdash. With this case report form, sponsors and medical. This article will discuss different case report. It supports healthcare professionals and.
These Templates Are Consistent With The Fda Cdash.
Web this article is an attempt to describe the methods of crf designing in clinical research and discusses the challenges encountered in this process. If you are looking to increase study start. Web the primary purpose of this case report form template is to organize and track vital information for clinical trials efficiently. Web in a case report form, you can track the unique changes of each research subject as the clinical trial progresses.
Web Gathering Resources For Case Report Forms Is A Great Place To Begin Optimizing Bottlenecks Within Your Clinical Trial Operations.
This application is developed by clindox, a supplier of. Web crfweb is an electronic case report form (ecrf) for capturing data in pharmaceutical and medical device clinical trials. With this case report form, sponsors and medical. Web electronic case report form initiative.
Web Case Report Forms (Crfs) Are Arguably The Most Important Documentation In A Clinical Trial Since They Are The Last Point Of Data Entry, Which Ultimately Influences The Outcome Of.
Web a case report form is a standardized questionnaire that is used during a clinical trial to collect data from each patient. Web a case report form (crf) is an electronic or paper document which is used in a clinical trial to record the protocol and required information about each participant. Web rdc case report form a controlled study of the ability of a traditional swedish smokeless tobacco product (“snus”) to. Web a case report form (or crf) is a paper or electronic questionnaire specifically used in clinical trial research.
Web This Page Provides Links To Commonly Used Clinical Trial Forms Relevant To Clinical Trials.
Web august 15, 2014 when creating crfs in a clinical study build, it’s important to ask the right questions, but it is equally important to ask in the right way. Web agaclinicaltrials while each of our research trials at aga clinical trials is unique in several ways, there are also some common medical trial elements that are. This article will discuss different case report. It supports healthcare professionals and.



![What Is a Case Report Form? [ Importance, Tips, Samples ]](https://images.sampleforms.com/wp-content/uploads/2018/02/Confidential-Case-Report-Form-in-PDF-1.jpg)
