Chapter 2 The Chemistry Of Life
Chapter 2 The Chemistry Of Life - If you are author or own the copyright of this. The chemistry of life study guide 5.0 (4 reviews) term 1 / 72 what are the subatomic particles that make up an atom? The chapter describes biochemical compounds and reactions as well as the significance of water to life. Web the chemistry of life chapter 2 the chemistry of life 2.1 atoms and molecules 2.2 chemical compounds 2.3 mixtures 2.4 chemical reactions 2.5 types of reactions 2.6 organic molecules 2.7 macromolecules needed for life 2.7 macromolecules needed for life. Introduction to the chemistry of life. 2.1 the nature of matter what 3 subatomic particles make up atoms? Web chemistry of life chapter 2. Web chapter 2 the chemistry of life study guide terms in this set (85) the main source of energy for living things carbohydrates help carry out chemical reactions proteins contain hydrogen, oxygen, nitrogen,. There are 3 videos in the series: For each of the following.
Web it covers atoms, elements, subatomic particles, chemical bonds, and chemical reactions. Click the card to flip 👆 definition 1 / 72 protons neutrons electrons click the card. If you are author or own the copyright of this. Water and carbon compounds play essential roles in organisms, which carry out chemical reactions in their daily life. 2 every object in the universe is composed in matter matter is anything that occupies space and has mass o found on earth in 3 physical states: Data analysis and presentation (instructor materials preparation) 2.1: Introduction to the chemistry of life. The chapter describes biochemical compounds and reactions as well as the significance of water to life. In this chapter, we will explore the chemistry of how atoms are. Web this chapter provides the chemistry background needed to understand the human body, its functions, and its processes.
If you are author or own the copyright of this. Introduction to the chemistry of life. Web section summaries/chapter 2 37 name_____ class_____ date _____ four groups of organic compounds found in living things are carbohydrates, lipids, nucleic acids, and proteins. Web the chemistry of life (chapter 2) chemical bonds join together the molecules and compounds of life. Data analysis and presentation (instructor materials preparation) 2.1: In your textbook, read about the role of carbon. Web the subatomic particles that make up atoms are protons, neutrons, and electrons. Figure 2.0 foods such as bread, fruit, and cheese are rich sources of biological macromolecules. The center of the atom that is formed by protons and neutrons bound together with strong forces. 2.1 the nature of matter what 3 subatomic particles make up atoms?
Biology Chapter 2 The Chemistry of Life
The elements carbon, hydrogen, nitrogen, oxygen, sulfur, and. There are 3 videos in the series: The chapter describes biochemical compounds and reactions as well as the significance of water to life. If you are author or own the copyright of this. 2.1 the nature of matter what 3 subatomic particles make up atoms?
PPT Chapter 2 Notes The Chemistry of Life PowerPoint Presentation
Removing energy (cooling) atoms and molecules decreases their motion,. The elements carbon, hydrogen, nitrogen, oxygen, sulfur, and. The video is shot in dr. The chapter describes biochemical compounds and reactions as well as the significance of water to life. Web start studying chapter 2:
Biology Chapter 2 The Chemistry Of Life Worksheet Answers —
Learn vocabulary, terms, and more with flashcards, games, and other study tools. In this chapter, we will explore the chemistry of how atoms are. Web start studying chapter 2: The chemistry of life study guide 5.0 (4 reviews) term 1 / 72 what are the subatomic particles that make up an atom? There are 3 videos in the series:
Chapter 2 The Chemistry Of Life Worksheet Answers —
In your textbook, read about the role of carbon. What are the basic chemical principles that affect living things? The center of the atom that is formed by protons and neutrons bound together with strong forces. Web the subatomic particles that make up atoms are protons, neutrons, and electrons. 2 every object in the universe is composed in matter matter.
PPT Chapter 2 Chemistry of Life PowerPoint Presentation, free
In your textbook, read about the role of carbon. Web the chemistry of life (chapter 2) chemical bonds join together the molecules and compounds of life. Web start studying chapter 2: Learn vocabulary, terms, and more with flashcards, games, and other study tools. Chemistry of life (instructor materials preparation) 2:
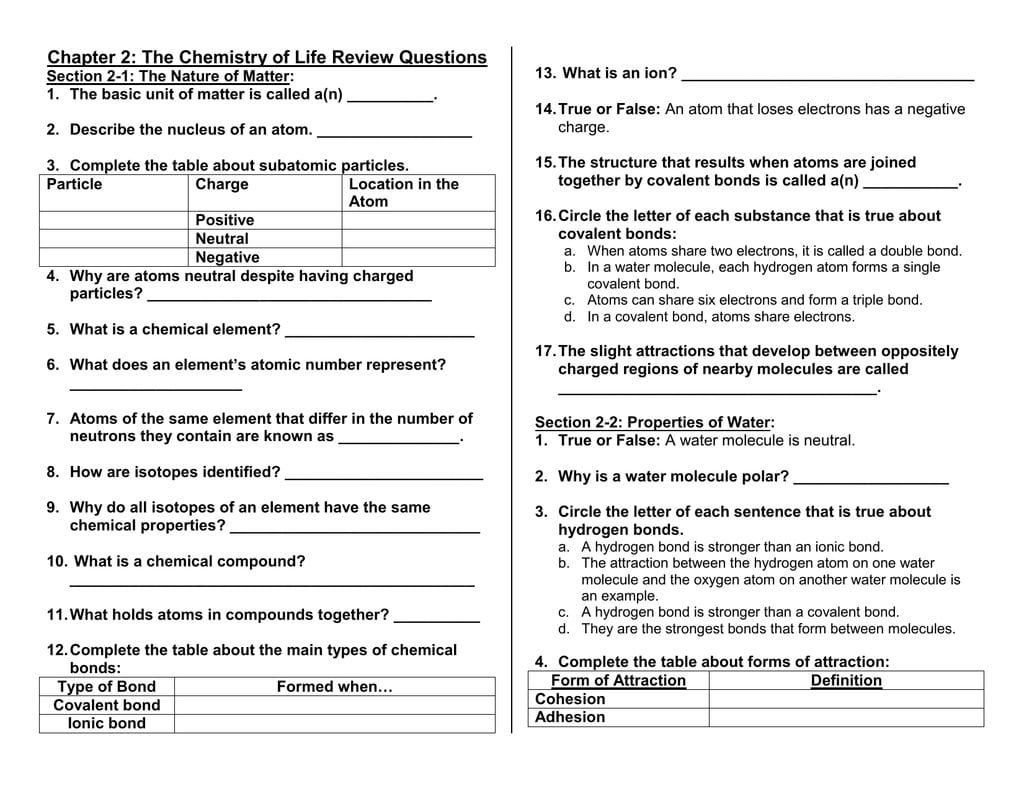
Chapter 2 The Chemistry Of Life Review Questions —
Learn vocabulary, terms, and more with flashcards, games, and other study tools. 2.1 the nature of matter what 3 subatomic particles make up atoms? The chapter describes biochemical compounds and reactions as well as the significance of water to life. Removing energy (cooling) atoms and molecules decreases their motion,. If you are author or own the copyright of this.
PPT Chapter 2 Chemistry of Life PowerPoint Presentation, free
The basic unit of matter. Learn vocabulary, terms, and more with flashcards, games, and other study tools. If you are author or own the copyright of this. The video is shot in dr. Web the subatomic particles that make up atoms are protons, neutrons, and electrons.
Biology Chapter 2 The Chemistry Of Life Worksheet Answers —
Gas, liquid, solid atoms are the. Water and carbon compounds play essential roles in organisms, which carry out chemical reactions in their daily life. For each of the following. Figure 2.0 foods such as bread, fruit, and cheese are rich sources of biological macromolecules. Web chapter 2 the chemistry of life study guide terms in this set (85) the main.
chapter 2 The chemistry of life Crossword WordMint
2 every object in the universe is composed in matter matter is anything that occupies space and has mass o found on earth in 3 physical states: Web the subatomic particles that make up atoms are protons, neutrons, and electrons. The elements carbon, hydrogen, nitrogen, oxygen, sulfur, and. The basic unit of matter. Web the chemistry of life chapter 2.
Chapter 2 Chemistry of Life
Web chemistry of life chapter 2. Data analysis and presentation (instructor materials preparation) 2.1: The center of the atom that is formed by protons and neutrons bound together with strong forces. The basic unit of matter. Removing energy (cooling) atoms and molecules decreases their motion,.
Web Chapter 2 Assessment The Chemistry Of Life Term 1 / 33 The Positively Charged Particle In An Atom Is Called The Click The Card To Flip 👆 Definition 1 / 33 Proton Click The Card To Flip 👆 Flashcards Learn Test Match Created By.
Web this chapter provides the chemistry background needed to understand the human body, its functions, and its processes. Click the card to flip 👆 definition 1 / 72 protons neutrons electrons click the card. The video is shot in dr. 2 every object in the universe is composed in matter matter is anything that occupies space and has mass o found on earth in 3 physical states:
Removing Energy (Cooling) Atoms And Molecules Decreases Their Motion,.
Web since organisms are made of elements arranged into molecule, the laws of physics and chemistry must apply to all biological processes. In your textbook, read about the role of carbon. For each of the following. There are 3 videos in the series:
The Elements Carbon, Hydrogen, Nitrogen, Oxygen, Sulfur, And.
In this chapter, we will explore the chemistry of how atoms are. This document was uploaded by user and they confirmed that they have the permission to share it. Web adding energy (heating) atoms and molecules increases their motion, resulting in an increase in temperature. The chapter describes biochemical compounds and reactions as well as the significance of water to life.
Data Analysis And Presentation (Instructor Materials Preparation) 2.1:
The basic unit of matter. The chemistry of life study guide 5.0 (4 reviews) term 1 / 72 what are the subatomic particles that make up an atom? Introduction to the chemistry of life. 2.1 the nature of matter what 3 subatomic particles make up atoms?