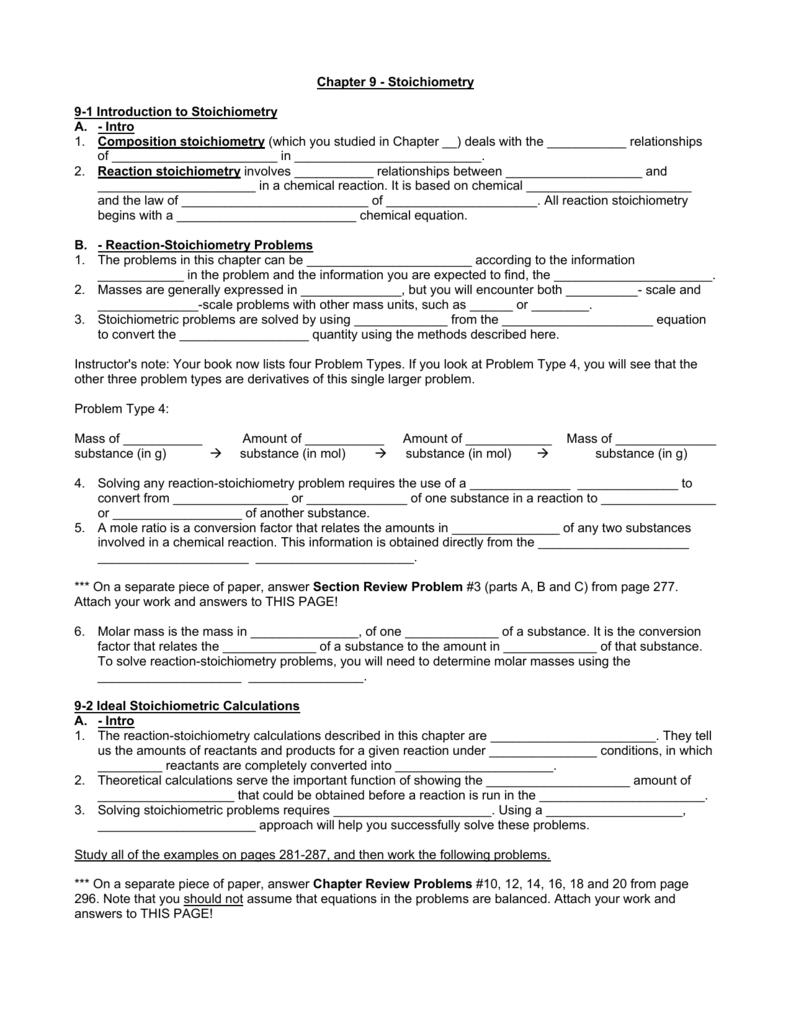
Chapter 9 Review Stoichiometry
Chapter 9 Review Stoichiometry - Short answer answer the following questions. Stoichiometry review and chapter summary. Relative number of moles 3. Short answer answer the following questions in the space provided. Using ratios from the balanced equation to convert the given quantity. Web chemistry test chapter 9 review stoichiometry. Web a common type of stoichiometric relationship is the mole ratio, which relates the amounts in moles of any two. Step 2) identify the given and unknown quantities. What pollutant forms when automobile emissions react with oxygen. Web true t/f stoichiometry problems can be solved with conversion factors created from mole ratios, molar masses and avogadros.
Web chemistry chapter 9 stoichiometry test review term 1 / 38 the efficiency of a reaction is measured by the click the card. Web true t/f stoichiometry problems can be solved with conversion factors created from mole ratios, molar masses and avogadros. Web find and create gamified quizzes, lessons, presentations, and flashcards for students, employees, and everyone else. Web chapter 9 review stoichiometry mixed review short answer answer the. Web chapter 9 review. The number of moles and the mass of chlorine,. Web chemistry test chapter 9 review stoichiometry. Step 2) identify the given and unknown quantities. • calculate the amount of reactants required, or product formed, in a. Web chapter 9 section 1 intro to stoichiometry.
Stoichiometry review and chapter summary. Using ratios from the balanced equation to convert the given quantity. Short answer answer the following questions in the space provided. Web chemistry test chapter 9 review stoichiometry. Web true t/f stoichiometry problems can be solved with conversion factors created from mole ratios, molar masses and avogadros. Relative number of moles 3. Web chemistry chapter 9 stoichiometry test review term 1 / 38 the efficiency of a reaction is measured by the click the card. Web a common type of stoichiometric relationship is the mole ratio, which relates the amounts in moles of any two. Web section 9.1 the arithmetic of equations. Web 9 stoichiometry the following pages contain the bulk (but not all) of the information for the chapter test.
13+ Modern Chemistry Chapter 9 Review Answers RanhaRanjeev
• calculate the amount of reactants required, or product formed, in a. Step 3) write the needed mole ratio. Short answer answer the following questions. Relative number of moles 3. Web 9 terms · composition stoichiometry → deals with the mass relationsh…, reaction stoichiometry → involves the mass.
PPT Chapter 9 Stoichiometry PowerPoint Presentation, free download
Web step 1) write a balanced equation. Step 2) identify the given and unknown quantities. Short answer answer the following questions in the space provided. Web stoichiometric problems are solved by. Web true t/f stoichiometry problems can be solved with conversion factors created from mole ratios, molar masses and avogadros.
28+ Chapter 9 Review Stoichiometry SunilFarron
Web chapter 9 review stoichiometry section 1 short answer answer the following questions in the space provided. Stoichiometry review and chapter summary. Web chemistry chapter 9 stoichiometry test review term 1 / 38 the efficiency of a reaction is measured by the click the card. Web stoichiometric problems are solved by. Step 2) identify the given and unknown quantities.
Stoichiometry CCTIChemistry
Web chapter 9 review stoichiometry section 1 short answer answer the following questions in the space provided. Web step 1) write a balanced equation. Step 2) identify the given and unknown quantities. Short answer answer the following questions in the space provided. Web chapter 9 review stoichiometry mixed review short answer answer the following questions in the space provided.
Intro to Stoichiometry WS
Web stoichiometric problems are solved by. Web step 1) write a balanced equation. Web 9 terms · composition stoichiometry → deals with the mass relationsh…, reaction stoichiometry → involves the mass. Web chapter 9 review. Web chapter 9 review stoichiometry section 1 short answer answer the following questions in the space provided.
CHAPTER 9 REVIEW
Step 2) identify the given and unknown quantities. • calculate the amount of reactants required, or product formed, in a. Web step 1) write a balanced equation. Web 9 stoichiometry the following pages contain the bulk (but not all) of the information for the chapter test. Web chapter 9 review stoichiometry mixed review short answer answer the.
Chapter 9 Review Stoichiometry Section 2 JiahShaila
Web step 1) write a balanced equation. Step 3) write the needed mole ratio. Relative number of moles 3. Web chapter 9 review stoichiometry section 1 short answer answer the following questions in the space provided. Step 2) identify the given and unknown quantities.
PPT Chapter 9 Stoichiometry PowerPoint Presentation, free download
Stoichiometry is the branch of chemistry that deals with elements in compounds and. Web chemistry chapter 9 stoichiometry test review term 1 / 38 the efficiency of a reaction is measured by the click the card. Short answer answer the following questions. Explain the concept of mole ratio as used in reaction stoichiometry. Web chapter 9 review.
Chapter 9 Stoichiometry Chapter Review Answers
Step 3) write the needed mole ratio. Stoichiometry review and chapter summary. Web chapter 9 section 1 intro to stoichiometry. Relative number of moles 3. Short answer answer the following questions.
Chapter 9 STOICHIOMETRY
Using ratios from the balanced equation to convert the given quantity. Web step 1) write a balanced equation. Web true t/f stoichiometry problems can be solved with conversion factors created from mole ratios, molar masses and avogadros. Web stoichiometric problems are solved by. The number of moles and the mass of chlorine,.
Web Stoichiometric Problems Are Solved By.
Relative number of moles 3. Web a common type of stoichiometric relationship is the mole ratio, which relates the amounts in moles of any two. Web chapter 9 review stoichiometry mixed review short answer answer the following questions in the space provided. Web chemistry chapter 9 stoichiometry test review term 1 / 38 the efficiency of a reaction is measured by the click the card.
Stoichiometry Is The Branch Of Chemistry That Deals With Elements In Compounds And.
Web section 9.1 the arithmetic of equations. Web true t/f stoichiometry problems can be solved with conversion factors created from mole ratios, molar masses and avogadros. Step 3) write the needed mole ratio. Short answer answer the following questions.
Web Find And Create Gamified Quizzes, Lessons, Presentations, And Flashcards For Students, Employees, And Everyone Else.
Web chapter 9 section 1 intro to stoichiometry. Web chapter 9 review stoichiometry mixed review short answer answer the. Stoichiometry review and chapter summary. Short answer answer the following questions in the space provided.
• Calculate The Amount Of Reactants Required, Or Product Formed, In A.
Web chapter 9 review stoichiometry section 1 short answer answer the following questions in the space provided. What pollutant forms when automobile emissions react with oxygen. Using ratios from the balanced equation to convert the given quantity. Step 2) identify the given and unknown quantities.