Clia Form 116
Clia Form 116 - Web 116 application, a clia number will be assigned. Web laboratory personnel report (clia) (for moderate and high complexity testing) form approved omb no. State of nc hiv testing, mam screening & pap screening applications. Web for questions regarding a clia certificate or fees: Web these items are required for initial application: List all personnel (e.g., laboratory assistant, phlebotomist,. Web all completed 116 applications and requests for corrections and/or changes can be emailed to: Web lab 116 (7/07) laboratory personnel report (continued) laboratory name or id number instructions: If applicable, please include the assigned clia. Clia has regulatory requirements for quality that all laboratories must meet.
Web listed on this form will not be reviewed, forwarded, or retained. Clia has regulatory requirements for quality that all laboratories must meet. If you have questions or concerns regarding where to submit your documents, please contact. Web these items are required for initial application: This clia number will allow laboratories to begin testing before a paper certifcate is mailed as long as applicable clia requirements. Web how do i enroll in or apply to the clia program? Web the missouri clia program oversees this certification program in our state and carries out certain inspections of laboratories on a routine basis, as well as investigating complaint. Web all completed 116 applications and requests for corrections and/or changes can be emailed to: Web laboratory personnel report (clia) (for moderate and high complexity testing) form approved omb no. If applicable, please include the assigned clia.
If you have questions or concerns regarding where to submit your documents, please contact. Web how do i enroll in or apply to the clia program? Hiv testing initial & renewal application (pdf, 81 kb) mammography. State of nc hiv testing, mam screening & pap screening applications. Web for questions regarding a clia certificate or fees: If applicable, please include the assigned clia. This clia number will allow laboratories to begin testing before a paper certifcate is mailed as long as applicable clia requirements. Web 116 application, a clia number will be assigned. Web any certification the clia issued from cms; Web lab 116 (7/07) laboratory personnel report (continued) laboratory name or id number instructions:
How to Apply for a CLIA Certificate? Filling out CMS116 Lighthouse
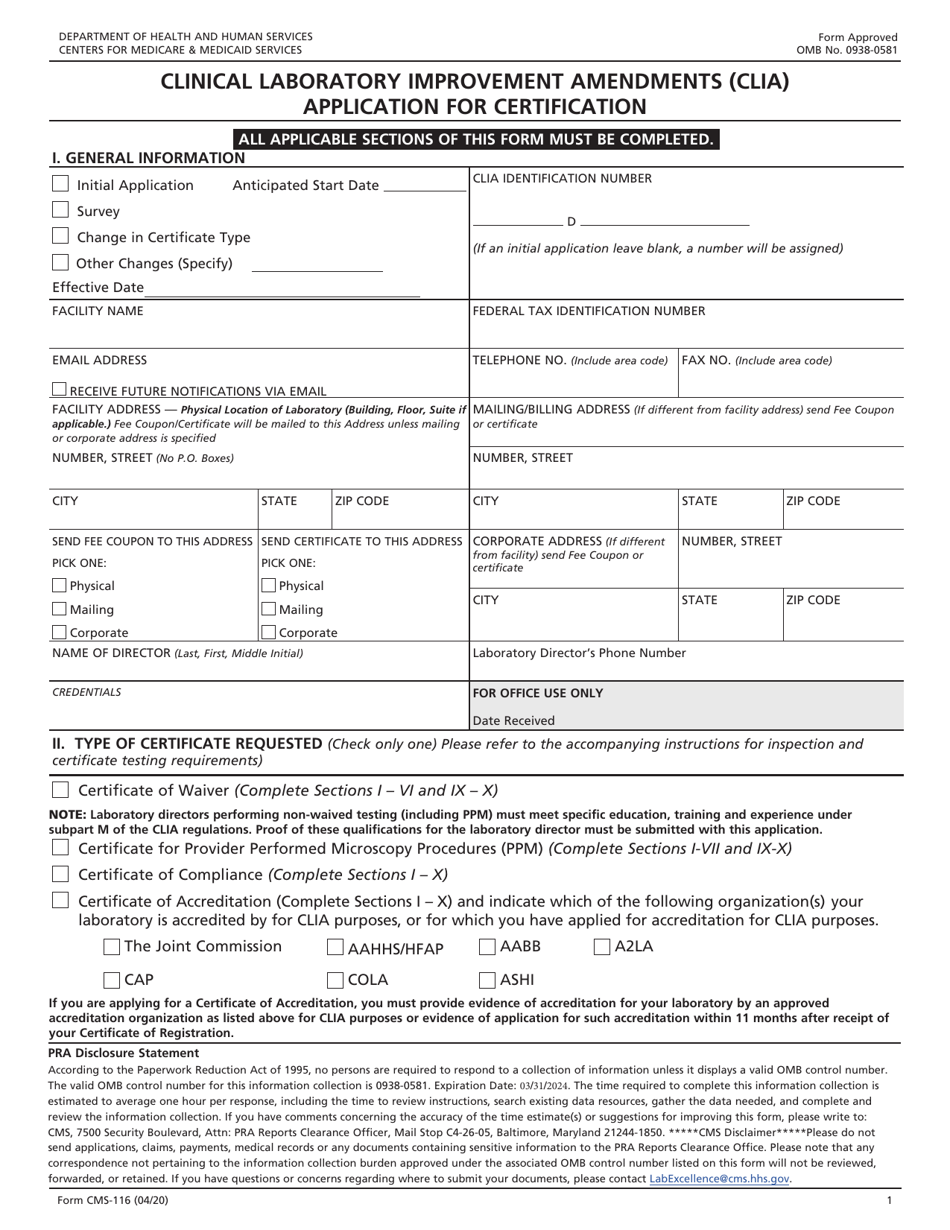
Web these items are required for initial application: Web any certification the clia issued from cms; Web 116 application, a clia number will be assigned. Web clinical laboratory professionals use the clia application cms 116 form to report certain information on performance of laboratory tests toclinical laboratory improvement. This guide helps laboratories seeking to apply for clia certification from cms.
CMS 116 Improved Standards for Clinical Laboratories pdfFiller Blog
List all personnel (e.g., laboratory assistant, phlebotomist,. If you have questions or concerns regarding where to submit your documents, please contact. If applicable, please include the assigned clia. Web cms form 116; Clia has regulatory requirements for quality that all laboratories must meet.
Form DV116 Download Fillable PDF or Fill Online Order on Request to
State of nc hiv testing, mam screening & pap screening applications. Web listed on this form will not be reviewed, forwarded, or retained. List all personnel (e.g., laboratory assistant, phlebotomist,. Web lab 116 (7/07) laboratory personnel report (continued) laboratory name or id number instructions: If applicable, please include the assigned clia.
Difference Between CLIA and ECLIA Compare the Difference Between
Web any certification the clia issued from cms; If applicable, please include the assigned clia. Web how do i enroll in or apply to the clia program? This guide helps laboratories seeking to apply for clia certification from cms. Hiv testing initial & renewal application (pdf, 81 kb) mammography.
Form 116 Download Fillable PDF or Fill Online Lien Release Form Montana
Web laboratory personnel report (clia) (for moderate and high complexity testing) form approved omb no. If you have questions or concerns regarding where to submit your documents, please contact. This clia number will allow laboratories to begin testing before a paper certifcate is mailed as long as applicable clia requirements. Web for questions regarding a clia certificate or fees: Web.
Fillable Form Cms116 Clinical Laboratory Improvement Amendments Of
Web clinical laboratory professionals use the clia application cms 116 form to report certain information on performance of laboratory tests toclinical laboratory improvement. This clia number will allow laboratories to begin testing before a paper certifcate is mailed as long as applicable clia requirements. Web 116 application, a clia number will be assigned. Web laboratory personnel report (clia) (for moderate.
Form CMS116 Download Fillable PDF or Fill Online Clinical Laboratory
List all personnel (e.g., laboratory assistant, phlebotomist,. Web any certification the clia issued from cms; Clia has regulatory requirements for quality that all laboratories must meet. Web 116 application, a clia number will be assigned. Web for questions regarding a clia certificate or fees:
Clia Application Cms 116 Form ≡ Fill Out Printable PDF Forms Online
Web any certification the clia issued from cms; If you have questions or concerns regarding where to submit your documents, please contact. This guide helps laboratories seeking to apply for clia certification from cms. Clia has regulatory requirements for quality that all laboratories must meet. Web for questions regarding a clia certificate or fees:
Form 116 California Installation Certification (Reusable PDF Format)
State of nc hiv testing, mam screening & pap screening applications. Web the missouri clia program oversees this certification program in our state and carries out certain inspections of laboratories on a routine basis, as well as investigating complaint. Web lab 116 (7/07) laboratory personnel report (continued) laboratory name or id number instructions: If you have questions or concerns regarding.
Clia Application Cms 116 Form ≡ Fill Out Printable PDF Forms Online
Web any certification the clia issued from cms; Clia has regulatory requirements for quality that all laboratories must meet. Web 116 application, a clia number will be assigned. Web for questions regarding a clia certificate or fees: Web all completed 116 applications and requests for corrections and/or changes can be emailed to:
This Clia Number Will Allow Laboratories To Begin Testing Before A Paper Certifcate Is Mailed As Long As Applicable Clia Requirements.
List all personnel (e.g., laboratory assistant, phlebotomist,. Web laboratory personnel report (clia) (for moderate and high complexity testing) form approved omb no. If applicable, please include the assigned clia. Web cms form 116;
Clia Has Regulatory Requirements For Quality That All Laboratories Must Meet.
State of nc hiv testing, mam screening & pap screening applications. Web clinical laboratory professionals use the clia application cms 116 form to report certain information on performance of laboratory tests toclinical laboratory improvement. Web lab 116 (7/07) laboratory personnel report (continued) laboratory name or id number instructions: Web how do i enroll in or apply to the clia program?
If You Have Questions Or Concerns Regarding Where To Submit Your Documents, Please Contact.
This guide helps laboratories seeking to apply for clia certification from cms. Web listed on this form will not be reviewed, forwarded, or retained. Web 116 application, a clia number will be assigned. Web all completed 116 applications and requests for corrections and/or changes can be emailed to:
Web Any Certification The Clia Issued From Cms;
Web the missouri clia program oversees this certification program in our state and carries out certain inspections of laboratories on a routine basis, as well as investigating complaint. Web for questions regarding a clia certificate or fees: Web these items are required for initial application: Hiv testing initial & renewal application (pdf, 81 kb) mammography.