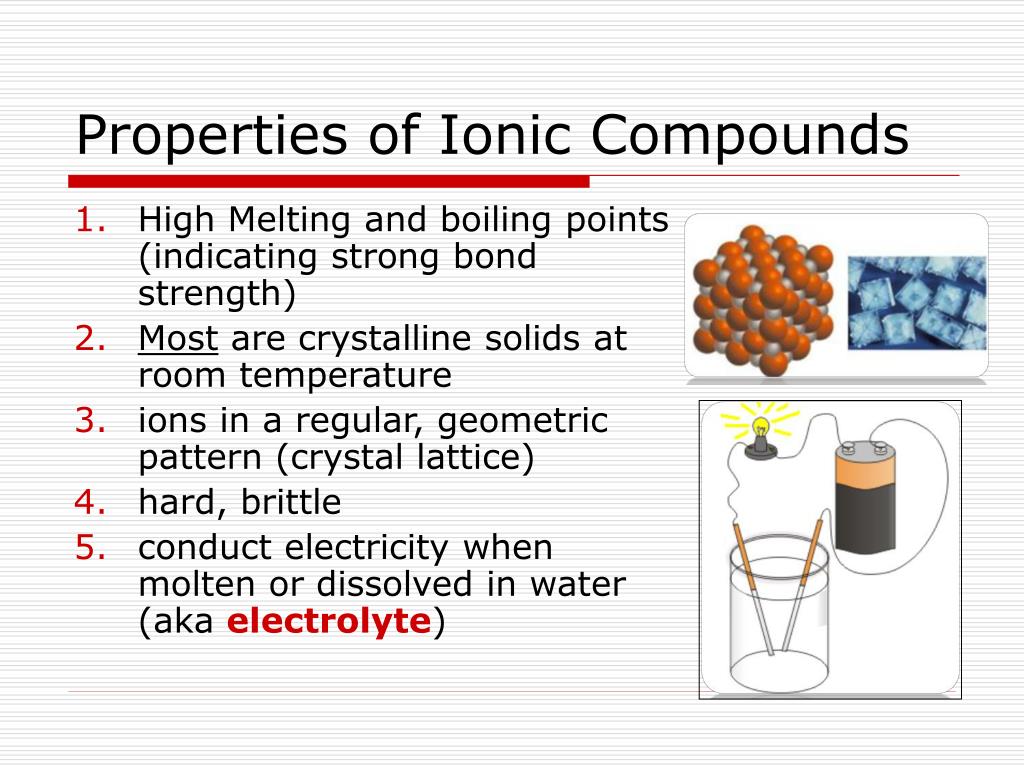
Describe How Ionic Compounds Form Crystals
Describe How Ionic Compounds Form Crystals - Web ionic compound definition. Sheri neva / getty images. Web in chemistry, an ionic crystal is a crystalline form of an ionic compound. Describe an ionic crystal, and explain why ionic crystals for different compounds might vary in shape. Oppositely charged ions of the ionic. Encyclopedia of physical science and technology (third. 11/21/2021 ionic compounds most of the rocks and minerals that surround us are made of ions held together through ionic bonding, the electrical. Briefly discuss three physical properties of ionic solids. Web however, ionic compounds do not exist as discrete molecules, as the dot diagrams may suggest. They are solids consisting of ions bound together by their electrostatic attraction into a regular lattice.
The ions have a regular, repeating arrangement called an ionic lattice. Ionic compounds can be defined as:. Web ionic compound definition. Sheri neva / getty images. Briefly discuss three physical properties of ionic solids. Web crystals are formed when the ionic liquids are cooled and get the hard ionic crystals under. Web ionic crystals are crystalline structures that grow from ionic bonds and are held together by electrostatic attraction. Web ionic crystals are aggregates of charged ions. Web however, ionic compounds do not exist as discrete molecules, as the dot diagrams may suggest. Ionic bonds are atomic bonds created by the.
Web in chemistry, an ionic crystal is a crystalline form of an ionic compound. The ions have a regular, repeating arrangement called an ionic lattice. Web however, ionic compounds do not exist as discrete molecules, as the dot diagrams may suggest. Encyclopedia of physical science and technology (third. This is why solid ionic compounds form crystals with regular shapes. Web ionic crystals are crystalline structures that grow from ionic bonds and are held together by electrostatic attraction. The opposite charges cancel out so ionic compounds have a net neutral. Web ionic compounds consist of oppositely charged ions that are held together by ionic bonds. These salts commonly exhibit ionic conductivity, which increases with temperature. Although molecular compounds form crystals, they frequently take other forms plus.
What Are The Properties Of Ionic Compound slidesharedocs
Web crystals are formed when the ionic liquids are cooled and get the hard ionic crystals under. The ions have a regular, repeating arrangement called an ionic lattice. Web the lattice arrangement continues in three dimensions. These salts commonly exhibit ionic conductivity, which increases with temperature. Ionic compounds can be defined as:.
Ionic Bonding Presentation Chemistry
Briefly discuss three physical properties of ionic solids. They can be of a single crystal or powdered form. Web ionic compound definition. Although molecular compounds form crystals, they frequently take other forms plus. These salts commonly exhibit ionic conductivity, which increases with temperature.
What Are The Properties Of Ionic Compound slidesharedocs
Web ionic compound definition. Web the lattice arrangement continues in three dimensions. Web crystals are formed when the ionic liquids are cooled and get the hard ionic crystals under. In order to minimize the potential energy of the system, ionic compounds take. Web crystals are formed when the ionic liquids are cooled and get the hard ionic crystals under.
What structural units make up ionic solids? Socratic
This is why solid ionic compounds form crystals with regular shapes. The opposite charges cancel out so ionic compounds have a net neutral. These salts commonly exhibit ionic conductivity, which increases with temperature. Web in chemistry, an ionic crystal is a crystalline form of an ionic compound. They can be of a single crystal or powdered form.
Properties of ionic compounds YouTube
These salts commonly exhibit ionic conductivity, which increases with temperature. Ionic bonds are atomic bonds created by the. They can be of a single crystal or powdered form. Web however, ionic compounds do not exist as discrete molecules, as the dot diagrams may suggest. Describe an ionic crystal, and explain why ionic crystals for different compounds might vary in shape.
The Crystal Lattice Structure of Ionic Compounds infographic diagram
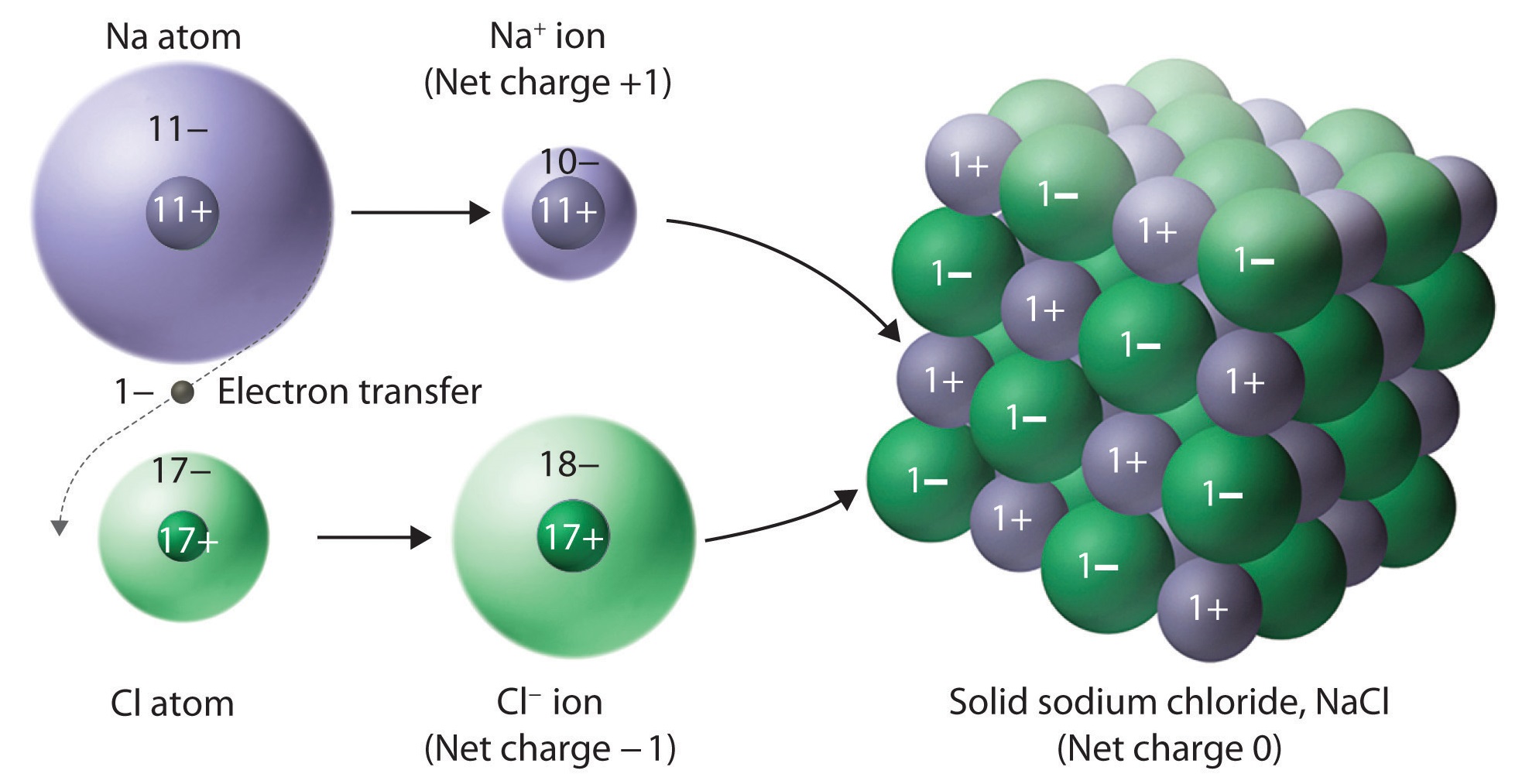
Nacl or salt is an example of an ionic compound. The opposite charges cancel out so ionic compounds have a net neutral. Web ionic compounds form a crystal lattice. Ionic compounds can be defined as:. Describe an ionic crystal, and explain why ionic crystals for different compounds might vary in shape.
Ionic Bond Definition, Types, Properties & Examples
Describe an ionic crystal, and explain why ionic crystals for different compounds might vary in shape. Web video test 1 2 3 4 the ionic lattice an ionic compound is a giant structure of ions. The opposite charges cancel out so ionic compounds have a net neutral. Web ionic compounds consist of oppositely charged ions that are held together by.
Ionic Bond Definition Easy Hard Science
Web video test 1 2 3 4 the ionic lattice an ionic compound is a giant structure of ions. Web in chemistry, an ionic crystal is a crystalline form of an ionic compound. They can be of a single crystal or powdered form. Describe an ionic crystal, and explain why ionic crystals for different compounds might vary in shape. Web.
Ionic Compound Properties
They are solids consisting of ions bound together by their electrostatic attraction into a regular lattice. Web ionic compounds form crystal lattices rather than amorphous solids. Briefly discuss three physical properties of ionic solids. Sheri neva / getty images. They can be of a single crystal or powdered form.
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare
Web video test 1 2 3 4 the ionic lattice an ionic compound is a giant structure of ions. This is why solid ionic compounds form crystals with regular shapes. The ions have a regular, repeating arrangement called an ionic lattice. These salts commonly exhibit ionic conductivity, which increases with temperature. They can be of a single crystal or powdered.
Web Ionic Crystals Are Aggregates Of Charged Ions.
Web however, ionic compounds do not exist as discrete molecules, as the dot diagrams may suggest. These salts commonly exhibit ionic conductivity, which increases with temperature. Ionic crystals consist of alternating cations and anions held together by electrostatic forces. They can be of a single crystal or powdered form.
Nacl Or Salt Is An Example Of An Ionic Compound.
In order to minimize the potential energy of the system, ionic compounds take. The ions have a regular, repeating arrangement called an ionic lattice. Describe an ionic crystal, and explain why ionic crystals for different compounds might vary in shape. Ionic bonds are atomic bonds created by the.
Web The Lattice Arrangement Continues In Three Dimensions.
Web video test 1 2 3 4 the ionic lattice an ionic compound is a giant structure of ions. Although molecular compounds form crystals, they frequently take other forms plus. Web ionic crystals are crystalline structures that grow from ionic bonds and are held together by electrostatic attraction. Sheri neva / getty images.
This Is Why Solid Ionic Compounds Form Crystals With Regular Shapes.
Ionic compounds can be defined as:. Web ionic compounds consist of oppositely charged ions that are held together by ionic bonds. The arrangement maximizes the attractive force between oppositely. The opposite charges cancel out so ionic compounds have a net neutral.

.PNG)




.PNG)