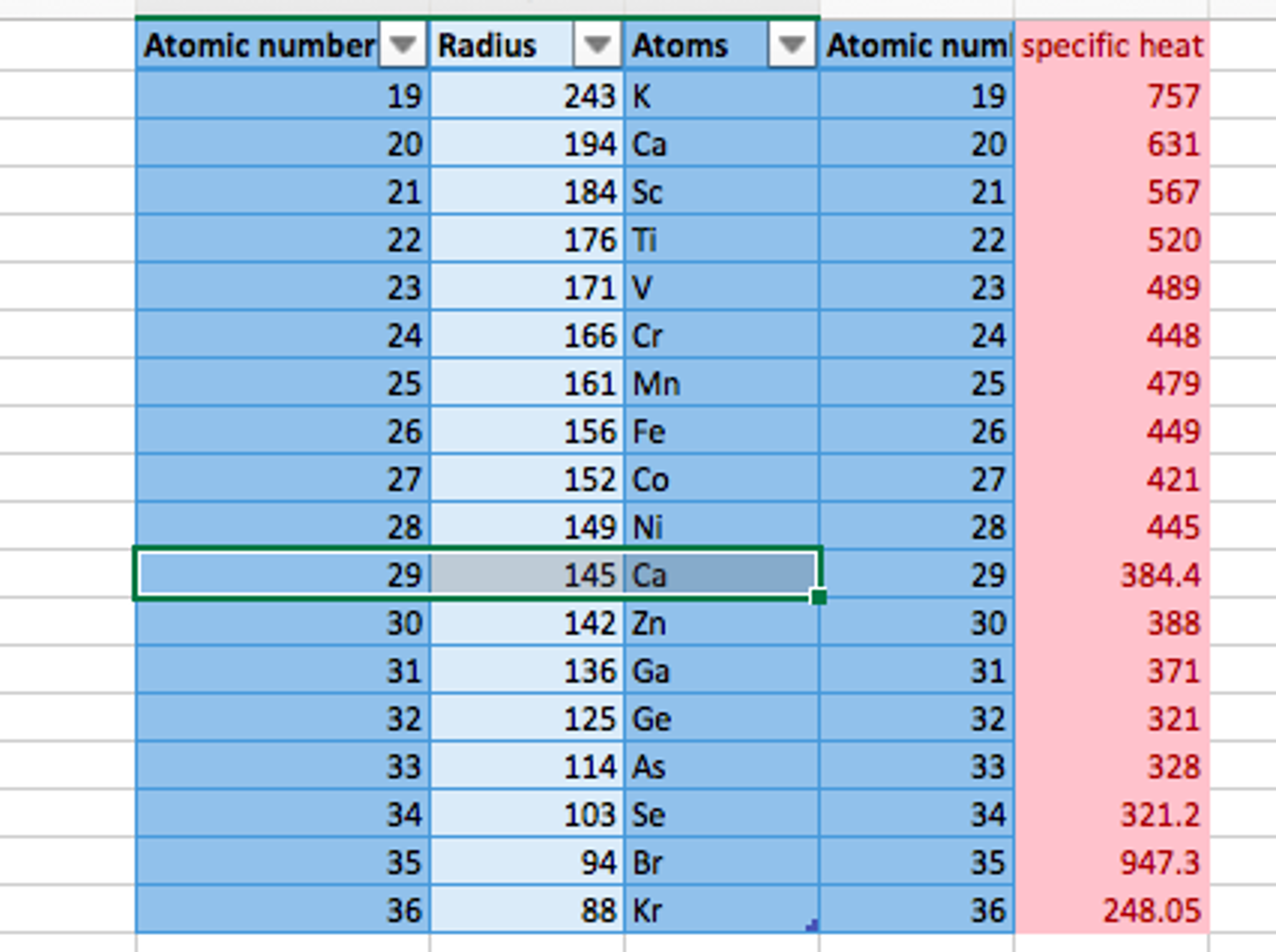
Do Metals Form Anions Or Cations
Do Metals Form Anions Or Cations - Oxygen often exists in a neutral state, but oxygen atoms tend to form. Loses 2 electrons to form 2+ ions. Web atoms gain electrons in their outer shell when they form negative ions, called anions. Web many elements can take the form of either anions or cations depending on the situation. Anions are negatively charged ions. Web most transition metals differ from the metals of groups 1, 2, and 13 in that they are capable of forming more than one cation with different ionic charges. Loses 1 electron to form 1+ ion. Web alkalis and alkali earth metals always tend to form cations whereas halogens always tend to form anions. Aluminum, a member of the iiia family, loses three electrons to form a 3+. Web group 1a and 2a of the periodic table, alkali metals and alkaline earth metals respectively, always form cations.
These ions are negative because they contain more electrons than protons. Loses 2 electrons to form 2+ ions. Web many elements can take the form of either anions or cations depending on the situation. In contrast, group 17a, which consists of halogens, always forms. Elements on the left side of the periodic table tend to make cations, compared to the right side of the periodic. Oxygen often exists in a neutral state, but oxygen atoms tend to form. Web atoms gain electrons in their outer shell when they form negative ions, called anions. Web alkaline metals on earth (the iia elements) lose two electrons to form a 2+ cation. In these compounds you have the cation, often an alkali metal itself, stabilized in a. Web most transition metals differ from the metals of groups 1, 2, and 13 in that they are capable of forming more than one cation with different ionic charges.
Web tara_donoghue terms in this set (60) what distinguishes a neutral atom from an ion ion has a different number of electrons do nonmetals form anions or cations? Elements on the left side of the periodic table tend to make cations, compared to the right side of the periodic. Aluminum, a member of the iiia family, loses three electrons to form a 3+. In these compounds you have the cation, often an alkali metal itself, stabilized in a. Web most transition metals differ from the metals of groups 1, 2, and 13 in that they are capable of forming more than one cation with different ionic charges. Web what do metals form? These ions are negative because they contain more electrons than protons. All metals will form cations. Web alkaline metals on earth (the iia elements) lose two electrons to form a 2+ cation. Web atoms gain electrons in their outer shell when they form negative ions, called anions.
17 Best images about What is an ion? on Pinterest Models, Shape and
Elements on the left side of the periodic table tend to make cations, compared to the right side of the periodic. These ions are negative because they contain more electrons than protons. Web cations are positively charged ions. In these compounds you have the cation, often an alkali metal itself, stabilized in a. Nonmetals will form anions, except for the.
Do metals form anions or cations quizlet? Book Vea
Web alkaline metals on earth (the iia elements) lose two electrons to form a 2+ cation. Web tara_donoghue terms in this set (60) what distinguishes a neutral atom from an ion ion has a different number of electrons do nonmetals form anions or cations? These ions are negative because they contain more electrons than protons. Elements on the left side.
PPT 1 Name the ions formed by these elements and classify them as
Web alkalis and alkali earth metals always tend to form cations whereas halogens always tend to form anions. Web a cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Web cations are positively charged ions. All metals will form cations. Web atoms gain electrons in.
Form a table of alkaline earth metal cations and the
Anions are negatively charged ions. Web many elements can take the form of either anions or cations depending on the situation. Nonmetals will form anions, except for the noble gases,. Web alkaline metals on earth (the iia elements) lose two electrons to form a 2+ cation. In contrast, group 17a, which consists of halogens, always forms.
Naming Simple Ionic Compounds Pathways to Chemistry
Web cations are positively charged ions. Web a cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Loses 2 electrons to form 2+ ions. Loses 1 electron to form 1+ ion. Web many elements can take the form of either anions or cations depending on.
anion Common anions, their names, formulas and the elements they are
Web tara_donoghue terms in this set (60) what distinguishes a neutral atom from an ion ion has a different number of electrons do nonmetals form anions or cations? Web a cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Web most transition metals differ from.
2.6 Ionic Compounds and Formulas Chemistry LibreTexts
Loses 2 electrons to form 2+ ions. Web tara_donoghue terms in this set (60) what distinguishes a neutral atom from an ion ion has a different number of electrons do nonmetals form anions or cations? Loses 1 electron to form 1+ ion. All metals will form cations. Web alkalis and alkali earth metals always tend to form cations whereas halogens.
PPT 1 Name the ions formed by these elements and classify them as
Web alkaline metals on earth (the iia elements) lose two electrons to form a 2+ cation. All metals will form cations. Aluminum, a member of the iiia family, loses three electrons to form a 3+. Loses 1 electron to form 1+ ion. These ions are negative because they contain more electrons than protons.
PPT Chapter 9 The Basics of Chemical Bonding PowerPoint Presentation
Web a cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Loses 1 electron to form 1+ ion. Oxygen often exists in a neutral state, but oxygen atoms tend to form. All metals will form cations. In these compounds you have the cation, often an.
Nonmetals and anion formation YouTube
Web alkalis and alkali earth metals always tend to form cations whereas halogens always tend to form anions. Elements on the left side of the periodic table tend to make cations, compared to the right side of the periodic. Web alkaline metals on earth (the iia elements) lose two electrons to form a 2+ cation. Web what do metals form?.
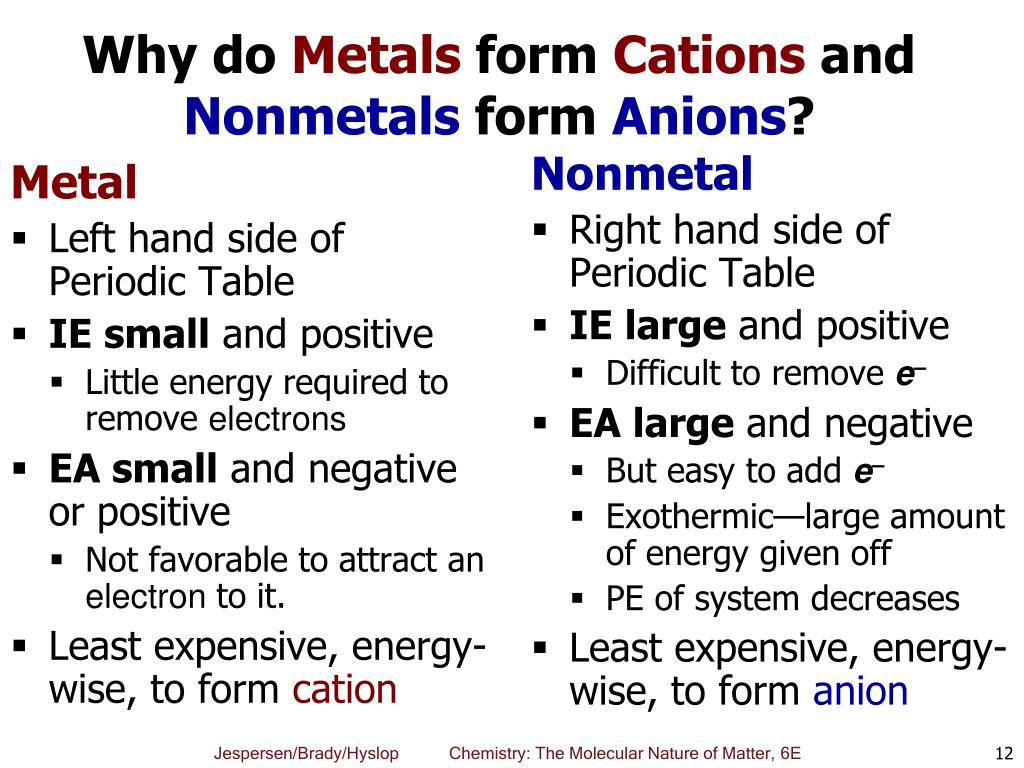
All Metals Will Form Cations.
These ions are negative because they contain more electrons than protons. Aluminum, a member of the iiia family, loses three electrons to form a 3+. Web alkalis and alkali earth metals always tend to form cations whereas halogens always tend to form anions. In these compounds you have the cation, often an alkali metal itself, stabilized in a.
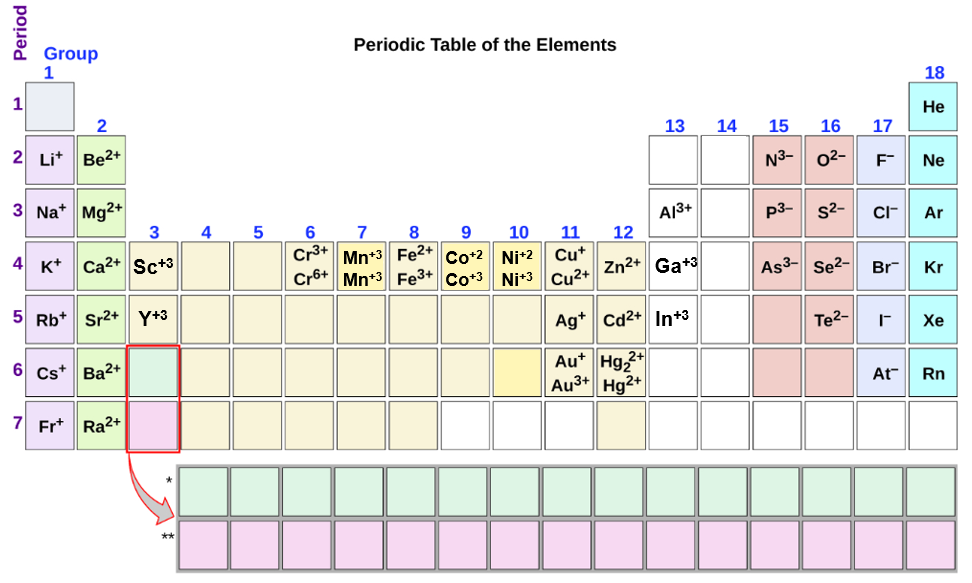
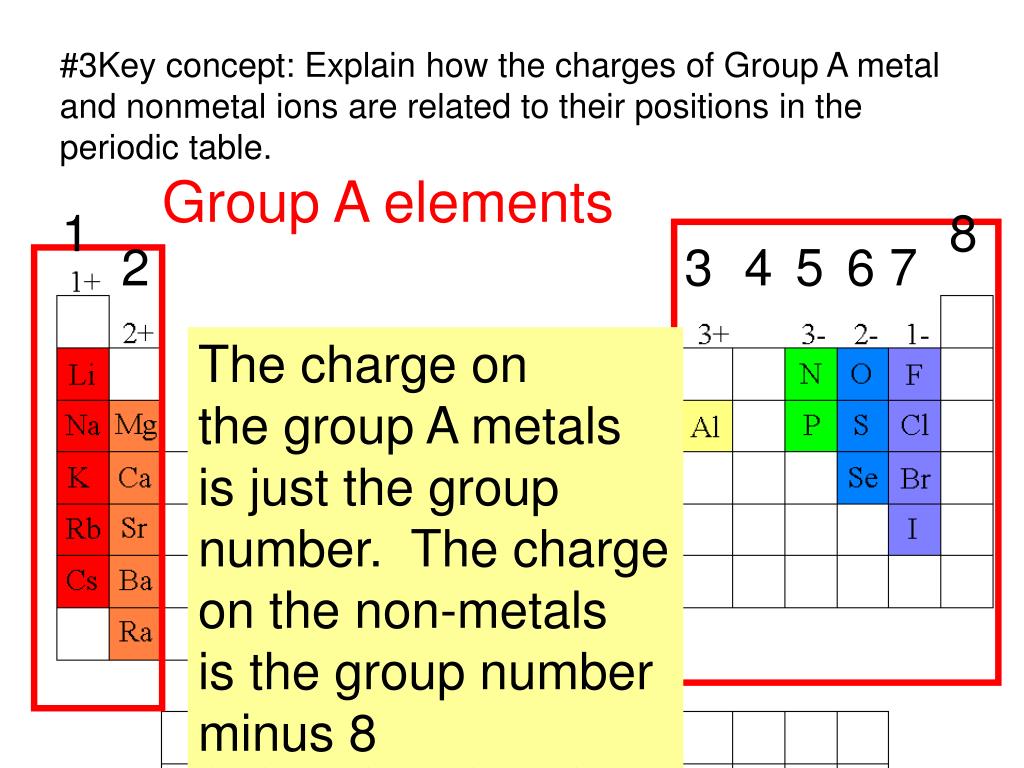
Web Group 1A And 2A Of The Periodic Table, Alkali Metals And Alkaline Earth Metals Respectively, Always Form Cations.
Oxygen often exists in a neutral state, but oxygen atoms tend to form. In contrast, group 17a, which consists of halogens, always forms. Loses 1 electron to form 1+ ion. Web alkaline metals on earth (the iia elements) lose two electrons to form a 2+ cation.
Web Tara_Donoghue Terms In This Set (60) What Distinguishes A Neutral Atom From An Ion Ion Has A Different Number Of Electrons Do Nonmetals Form Anions Or Cations?
Web most transition metals differ from the metals of groups 1, 2, and 13 in that they are capable of forming more than one cation with different ionic charges. Web atoms gain electrons in their outer shell when they form negative ions, called anions. Web what do metals form? Loses 2 electrons to form 2+ ions.
Web Cations Are Positively Charged Ions.
Web a cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Web many elements can take the form of either anions or cations depending on the situation. Nonmetals will form anions, except for the noble gases,. Elements on the left side of the periodic table tend to make cations, compared to the right side of the periodic.