Elements Exist In Which Form Of Matter
Elements Exist In Which Form Of Matter - Matter is usually classified into three classical states, with plasma sometimes added as a fourth. Web updated on april 02, 2020 what is an element? Freezing point temperature at which a liquid. On earth, matter commonly exists in three states: In physics, degenerate refers to two states that have the same energy and are thus interchangeable. Solids, of fixed shape and volume; There are 118 known elements. The three most common states are known as solid, liquid and gas. Web matter typically exists in one of three states: Atoms themselves are made up of even smaller bits of.
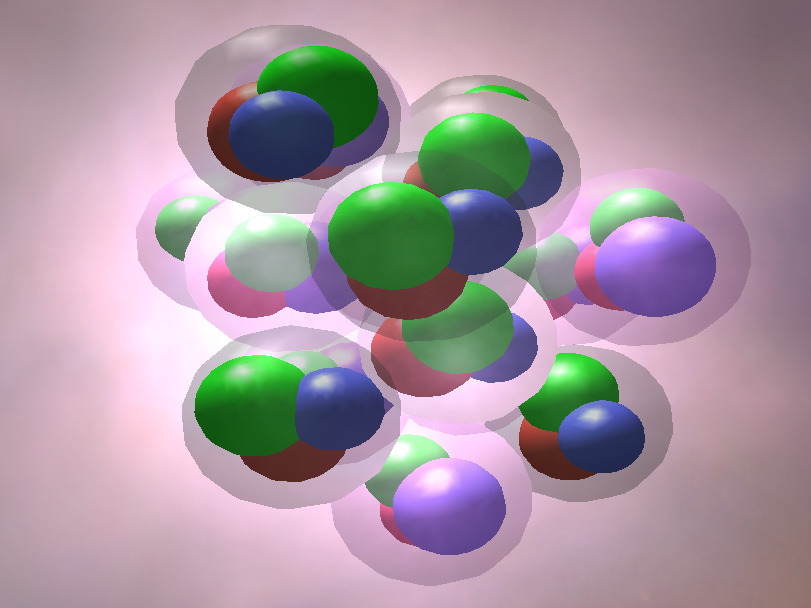
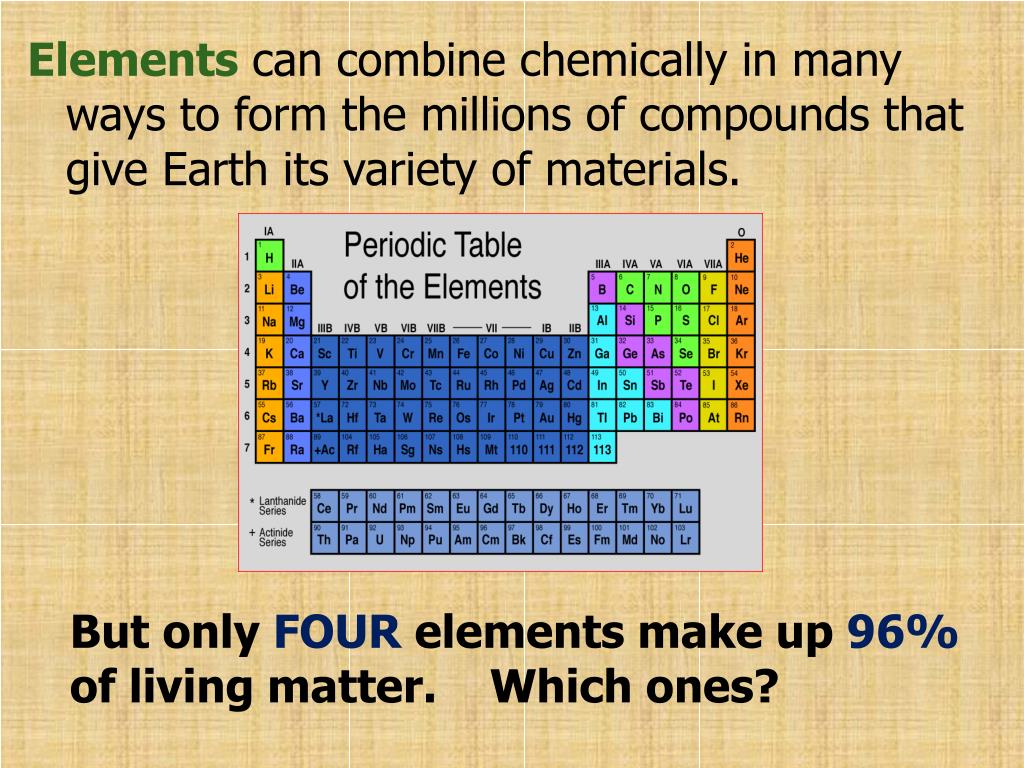
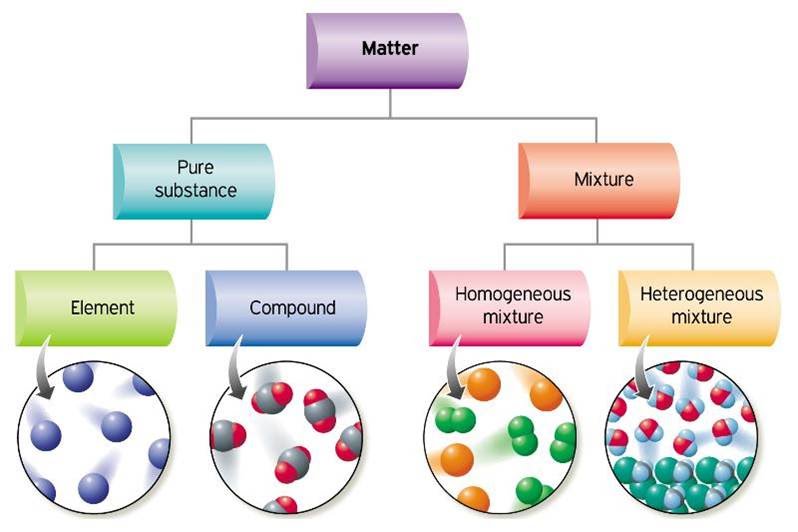
Web elements exist in which form of matter? All of the above d. Web elements may be thought of as the basic chemical building blocks of matter. Web natural science is concerned with things that change, and aristotle divides changes into two main types: There are accidental changes, which involve concrete. Atoms themselves are made up of even smaller bits of. Web the most familiar forms of matter — elements and compounds —are made of up tiny particles called atoms. Web note that an element: Web in many substances, atoms are combined into molecules. Web the elements that exist as diatomic molecules are hydrogen (h 2), nitrogen (n 2), fluorine (f 2), oxygen (o 2), iodine (i 2), chlorine (cl 2) and bromine (br 2).
There are 118 known elements. Matter is made up of atoms that are indivisible and indestructible. Degenerate matter is supported by the pauli exclusion principle, which prevents two fermionic particles from occupying the same quantu… Atoms themselves are made up of even smaller bits of. Web updated on april 02, 2020 what is an element? All of the above **** measureing which physical property is most likely to produce the most precise results. Web depending on temperature and some other factors, matter can exist in several states. Web the most familiar forms of matter — elements and compounds —are made of up tiny particles called atoms. Web in many substances, atoms are combined into molecules. Web the elements that exist as diatomic molecules are hydrogen (h 2), nitrogen (n 2), fluorine (f 2), oxygen (o 2), iodine (i 2), chlorine (cl 2) and bromine (br 2).
Elements of Matter Fooya Films
Liquids, of variable shape but. Matter is usually classified into three classical states, with plasma sometimes added as a fourth. Web matter typically exists in one of three states: The three most common states are known as solid, liquid and gas. Web the elements that exist as diatomic molecules are hydrogen (h 2), nitrogen (n 2), fluorine (f 2), oxygen.
Grade 5 Elements/Matter
Web elements may be thought of as the basic chemical building blocks of matter. Freezing point temperature at which a liquid. Web elements exist in which form of matter? Under extremely high pressure, as in the cores of dead stars, ordinary matter undergoes a transition to a series of exotic states of matter collectively known as degenerate matter, which are.
PPT Matter & the Elements Important to Life PowerPoint Presentation
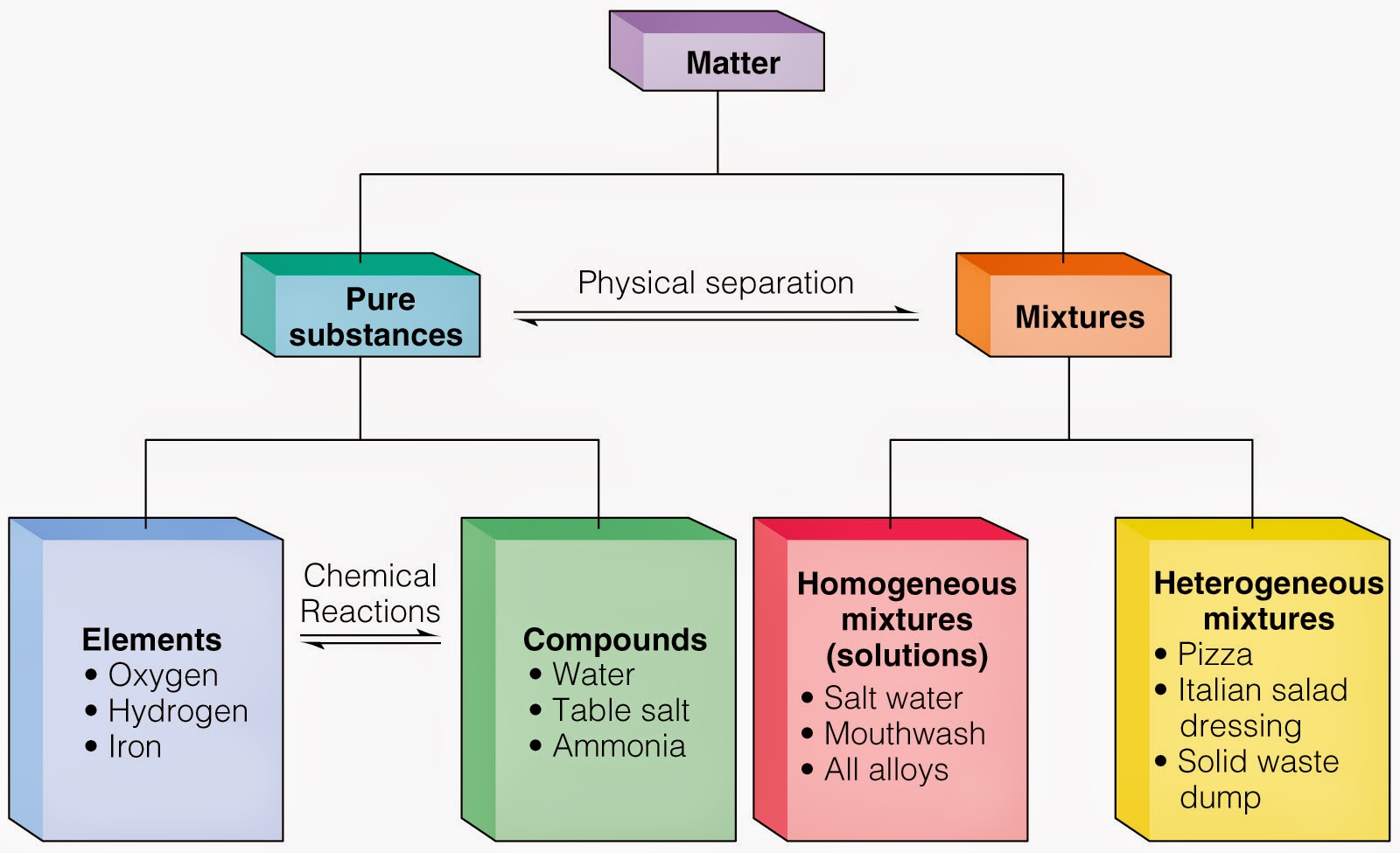
Degenerate matter is supported by the pauli exclusion principle, which prevents two fermionic particles from occupying the same quantu… Web states of matter are the different forms that elements, compounds and mixtures will exist in as either solids, liquids or gases depending on how close their particles are. Consists of only one kind of atom, cannot be broken down into.
How many Elements can there be? YouTube
Liquids, of variable shape but. Atoms of different elements have different weights and different. Web elements may be thought of as the basic chemical building blocks of matter. Atoms themselves are made up of even smaller bits of. Web the most familiar forms of matter — elements and compounds —are made of up tiny particles called atoms.
What temperature range are most elements on the periodic table liquids
In physics, degenerate refers to two states that have the same energy and are thus interchangeable. Web natural science is concerned with things that change, and aristotle divides changes into two main types: Web in many substances, atoms are combined into molecules. There are accidental changes, which involve concrete. Matter is usually classified into three classical states, with plasma sometimes.
Elements of Matter available now Fooya Films
Web matter typically exists in one of three states: On earth, matter commonly exists in three states: Matter is usually classified into three classical states, with plasma sometimes added as a fourth. A chemical element is the simplest form of matter that cannot be broken down using any chemical means. In physics, degenerate refers to two states that have the.
ALCHEMIST FREESTYLE STATE OF MATTER
Web the most familiar forms of matter — elements and compounds —are made of up tiny particles called atoms. These different forms are called isotopes , and are created when an element. The three most common states are known as solid, liquid and gas. Web while an element has a unique number of protons, each element can also exist in.
Elements of Properties of Matter by D.S. Mathur
Web updated on april 02, 2020 what is an element? Freezing point temperature at which a liquid. Web the elements that exist as diatomic molecules are hydrogen (h 2), nitrogen (n 2), fluorine (f 2), oxygen (o 2), iodine (i 2), chlorine (cl 2) and bromine (br 2). Web depending on temperature and some other factors, matter can exist in.
6th Grade Science 5th Six Weeks (Wk 1 & 2) Matter Pure Substances and
Web elements exist in which form of matter? Web while an element has a unique number of protons, each element can also exist in different forms. Consists of only one kind of atom, cannot be broken down into a simpler type of matter by either physical or chemical means, and; Matter is made up of atoms that are indivisible and.
Web States Of Matter Are The Different Forms That Elements, Compounds And Mixtures Will Exist In As Either Solids, Liquids Or Gases Depending On How Close Their Particles Are.
A chemical element is the simplest form of matter that cannot be broken down using any chemical means. Each element is identified according to the number of. Web natural science is concerned with things that change, and aristotle divides changes into two main types: Solids, of fixed shape and volume;
There Are Accidental Changes, Which Involve Concrete.
Web in many substances, atoms are combined into molecules. Matter is usually classified into three classical states, with plasma sometimes added as a fourth. Luster how shiny a material is. Web elements may be thought of as the basic chemical building blocks of matter.
Web Matter Typically Exists In One Of Three States:
In physics, degenerate refers to two states that have the same energy and are thus interchangeable. Web the most familiar forms of matter — elements and compounds —are made of up tiny particles called atoms. Atoms of different elements have different weights and different. Matter is made up of atoms that are indivisible and indestructible.
Freezing Point Temperature At Which A Liquid.
All of the above d. Atoms themselves are made up of even smaller bits of. All atoms of an element are identical. All of the above **** measureing which physical property is most likely to produce the most precise results.