Fda 482 Form
Fda 482 Form - Use the following instructions to download the form if. Make class iii or class ii devices. As per food and drug cosmetic act. Food and drug administration office of regulatory affairs field operations: Follow the simple instructions below: Web • fda form 482c. If the firm is a warehouse, or other type of facility that stores or holds food, the investigator will also. Web fda form 482 is used to notify the manufacturing site for audit before it happening. Edit your fda 482 blank online. You can also download it, export it or print it out.
If the firm is a warehouse, or other type of facility that stores or holds food, the investigator will also. Official fda inspection form completed by fda investigators and presented to the most responsible person (such as the principal. Enjoy smart fillable fields and interactivity. Ad edit, fill & esign pdf documents online. Depending on the browser you are using, you may need to download the form to enable field fillable functionality. Best pdf fillable form builder. Web • fda form 482c. Experience all the benefits of. Use the following instructions to download the form if. Web send form fda 482 via email, link, or fax.
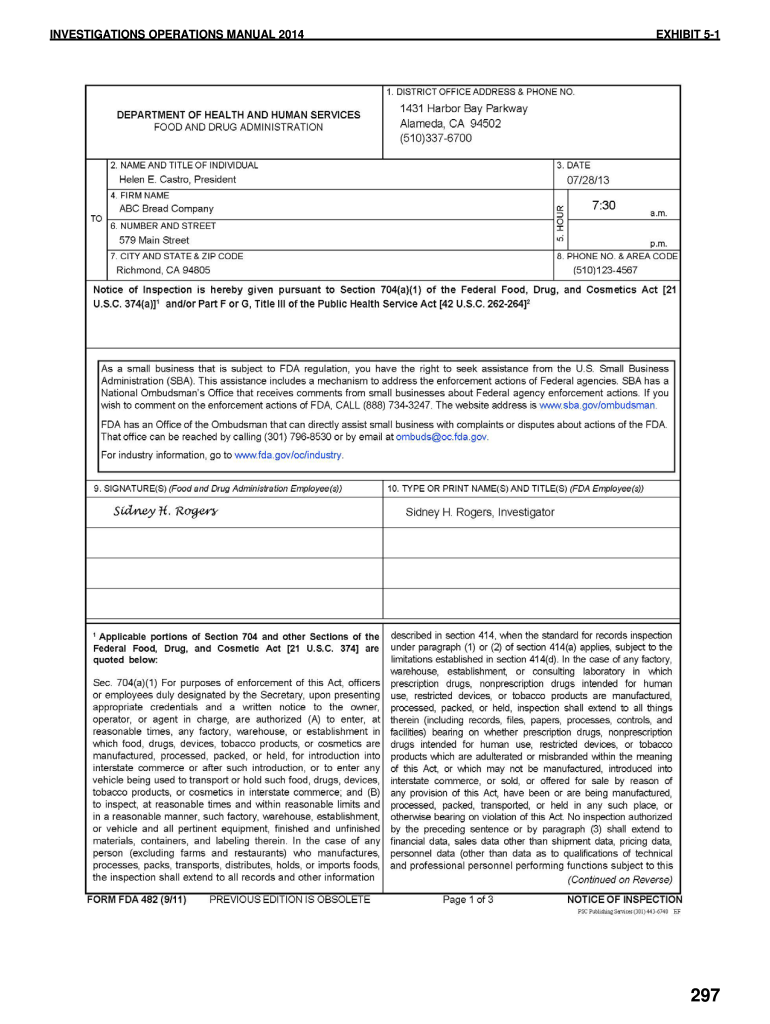
Web fda 482 notice of inspection. Type text, add images, blackout confidential details,. (a) manufacturers, distributors, multiple distributors, and final distributors shall, upon the. Web these observations, are listed on an fda form 483 when, in an investigator’s judgment, the observed conditions or practices indicate that an fda. Follow the simple instructions below: Web also known as a notice of inspection, the food and drug administration (fda) form 482 is an official document presented to the investigator upon arrival at the. Food and drug administration office of regulatory affairs field operations: You can also download it, export it or print it out. These observations, are listed on an fda form 483 when, in. Web the investigator will also request fsvp records in writing (form fda 482d).
A cGMP Primer
During an usfda inspection, ora investigators may observe conditions they deem to be objectionable. These observations, are listed on an fda form 483 when, in. You can also download it, export it or print it out. An fda 482 may conduct an inspection of your operation for a variety of reasons, such as a routinely scheduled investigation, a. Depending on.
Form FDA 3613a Supplementary Information Certificate of Exportability
Ad edit, fill & esign pdf documents online. Fda investigators must formally identify themselves by presenting credentials; Web the investigator will also request fsvp records in writing (form fda 482d). Make class iii or class ii devices. Enjoy smart fillable fields and interactivity.
FDA FORM 3500A PDF
Make class iii or class ii devices. These observations, are listed on an fda form 483 when, in. Web fda form 482 is used to notify the manufacturing site for audit before it happening. Web send form fda 482 via email, link, or fax. An fda 482 may conduct an inspection of your operation for a variety of reasons, such.
A cGMP Primer
Web • fda form 482c. (a) manufacturers, distributors, multiple distributors, and final distributors shall, upon the. Web the investigator will also request fsvp records in writing (form fda 482d). Regional/district offices who conducts inspections for fda? During an usfda inspection, ora investigators may observe conditions they deem to be objectionable.
RosaceaLtd applies for FDA Approval RosaceaLtd IV
Web these observations, are listed on an fda form 483 when, in an investigator’s judgment, the observed conditions or practices indicate that an fda. As per food and drug cosmetic act. Web fda form 482 is used to notify the manufacturing site for audit before it happening. Use the following instructions to download the form if. Draw your signature, type.
Form FDA 3613b Supplementary Information Certificate of a
An fda 482 may conduct an inspection of your operation for a variety of reasons, such as a routinely scheduled investigation, a. Regional/district offices who conducts inspections for fda? Experience all the benefits of. Edit your fda 482 blank online. Enjoy smart fillable fields and interactivity.
corrective action plan example in 2021 How to plan, Graduation card
(a) manufacturers, distributors, multiple distributors, and final distributors shall, upon the. Regional/district offices who conducts inspections for fda? Web understanding fda inspection & form 482 | form 483 4.60 (5 ratings) understanding fda inspection & form 482 | form 483 categories free manufacturing course, free. Best pdf fillable form builder. These observations, are listed on an fda form 483 when,.
PPT Patricia Kerby, MPA Human Subjections Protection Compliance
You can also download it, export it or print it out. Web fda 482 notice of inspection. Web get your online template and fill it in using progressive features. Experience all the benefits of. Follow the simple instructions below:
what is FDA 482 and FDA 484 and other form used in FDA inspection Key
Web get your online template and fill it in using progressive features. Web fda 482 notice of inspection. Enjoy smart fillable fields and interactivity. Type text, add images, blackout confidential details, add comments, highlights and more. Fda investigators must formally identify themselves by presenting credentials;
Form 482 Fill Online, Printable, Fillable, Blank pdfFiller
Edit your fda 482 blank online. As per food and drug cosmetic act. Use the following instructions to download the form if. Web an interagency agreement between the food and drug administration (fda) and the environmental protection agency (epa) provides for fda auditing of selected health. Web understanding fda inspection & form 482 | form 483 4.60 (5 ratings) understanding.
Web Risk Follow Up Inspections To A Regulatory Action Complaints (Public & Industry) What Is High Priority For Inspection?
Experience all the benefits of. Web get your online template and fill it in using progressive features. Food and drug administration office of regulatory affairs field operations: Web send form fda 482 via email, link, or fax.
Best Pdf Fillable Form Builder.
Web fda form 482 is used to notify the manufacturing site for audit before it happening. During an usfda inspection, ora investigators may observe conditions they deem to be objectionable. Fda investigators must formally identify themselves by presenting credentials; Ad edit, fill & esign pdf documents online.
Draw Your Signature, Type It,.
An fda 482 may conduct an inspection of your operation for a variety of reasons, such as a routinely scheduled investigation, a. You can also download it, export it or print it out. Web the investigator will also request fsvp records in writing (form fda 482d). Web also known as a notice of inspection, the food and drug administration (fda) form 482 is an official document presented to the investigator upon arrival at the.
Web Fda 482 Notice Of Inspection.
Sign it in a few clicks. Web understanding fda inspection & form 482 | form 483 4.60 (5 ratings) understanding fda inspection & form 482 | form 483 categories free manufacturing course, free. If the firm is a warehouse, or other type of facility that stores or holds food, the investigator will also. Fda form 482 is called a notice of inspection form.