How Many Bonds Does Sulfur Form
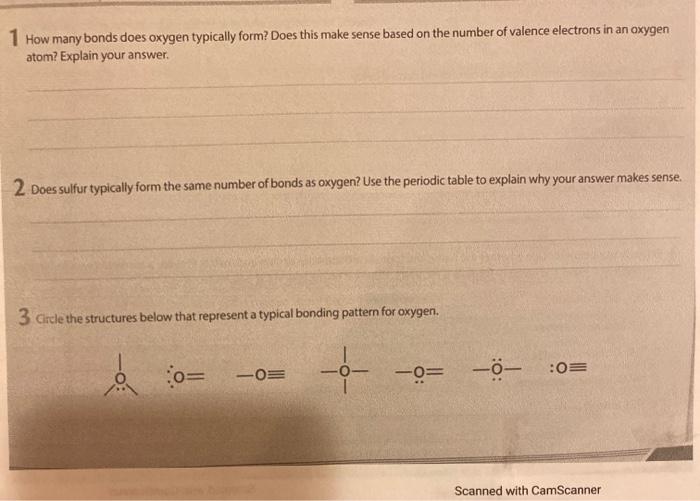
How Many Bonds Does Sulfur Form - Web sulfate is 4 single bonds. The octet rule states that atoms of all elements forms bond with other atoms in such a way that each atom has eight electrons (octet) in its. Web sulfur is in group 6a of the periodic table. Web so, sulfur and phosphorus really can form additional bonds. Sulfur can make 2, 4 or 6 bonds. This is because of its 3p4 orbital. It can do this by forming. Web 1 answer brian m. 2 how many electrons are shared in a double bond? Web sulphur hexafluoride ( sf 6) is an example of a compound where sulphur has six bonds.
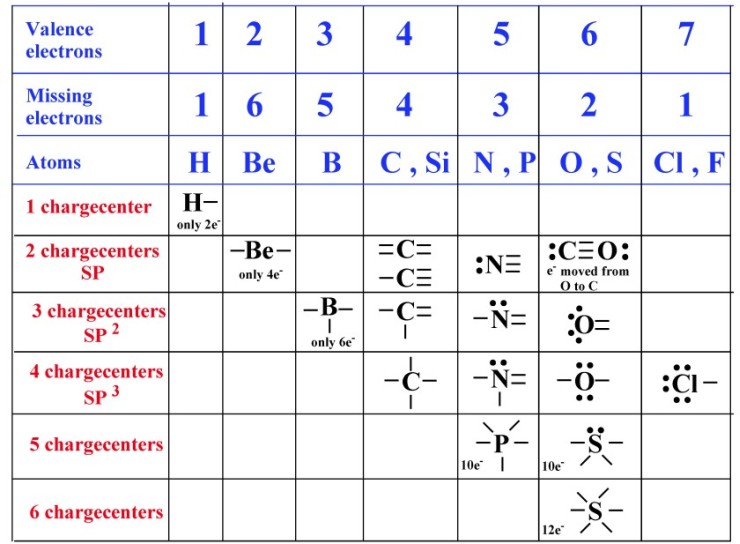
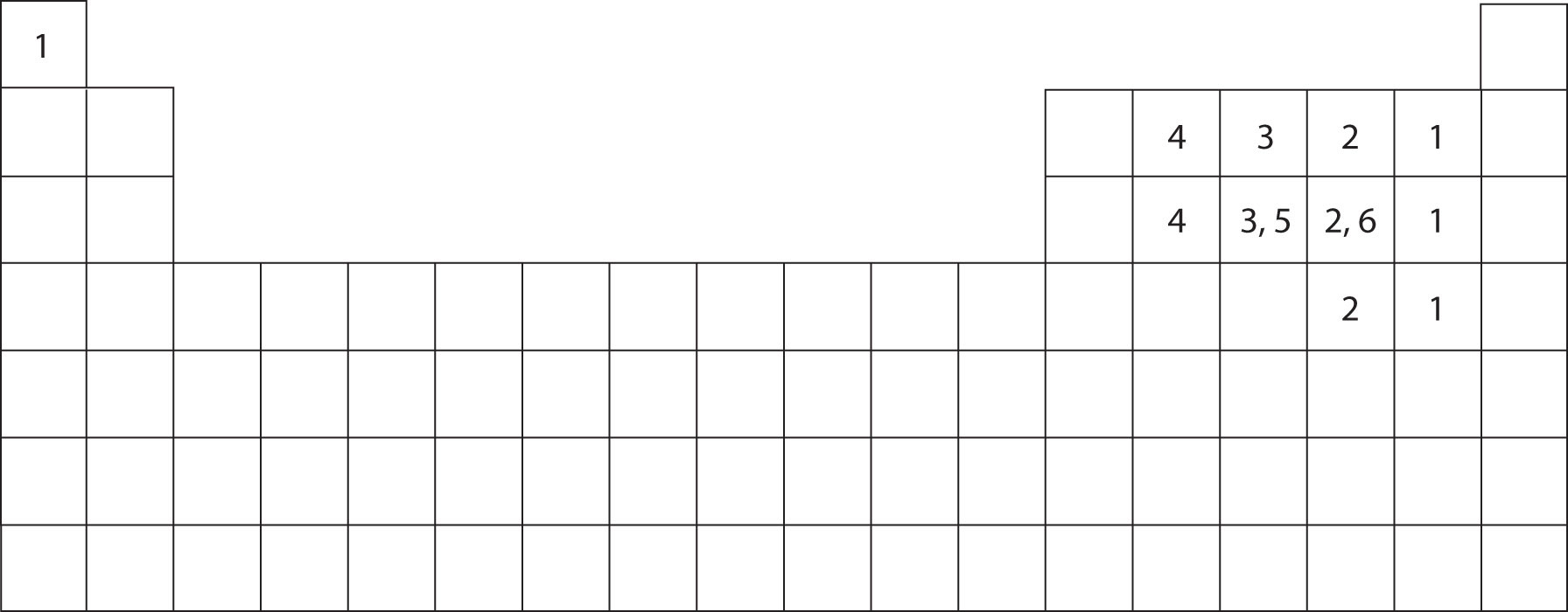
Web sulfur is in group 6a of the periodic table. It is in the same column of the periodic table of elements as oxygen is and oxygen will form two bonds. Web in each case, the sum of the number of bonds and the number of lone pairs is 4, which is equivalent to eight (octet) electrons. Sulfur is capable of forming 6 bonds because it can have an expanded valence shell; Web how many bonds can sulfur have, sulfur can make use of its 2 unpaired electrons to form 2 covalent bonds plus the 4 electrons from its 2 lone pairs to give a. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. Following the octet rule, how many covalent bonds can sulfur form? Sulfur is in period 3 of the periodic table. Web so, sulfur and phosphorus really can form additional bonds. Web up to 6% cash back sulfur is a nonmetal in group 6a , and therefore has 6 valence electrons.
Web sulfur is in group 6a of the periodic table. Table showing 4 different atoms, each. 2 how many electrons are shared in a double bond? Web sulfate is 4 single bonds. It is in the same column of the periodic table of elements as oxygen is and oxygen will form two bonds. It can do this by forming. Sulfur is capable of forming 6 bonds because it can have an expanded valence shell; Web how many chemical bonds can a sulfur atom make? In order to obey the octet rule, it needs to gain 2 electrons. Sulfur is in period 3 of the periodic table.
LabXchange
Sulfur is capable of forming 6 bonds because it can have an expanded valence shell; 2 how many electrons are shared in a double bond? Following the octet rule, how many covalent bonds can sulfur form? It is in the same column of the periodic table of elements as oxygen is and oxygen will form two bonds. The bond length.
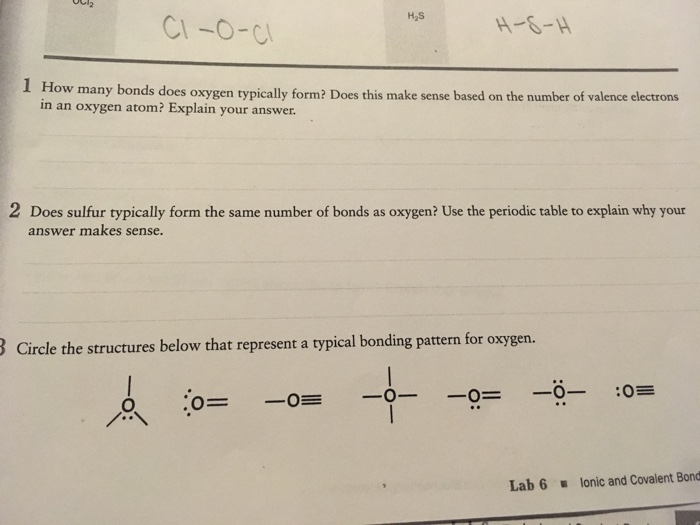
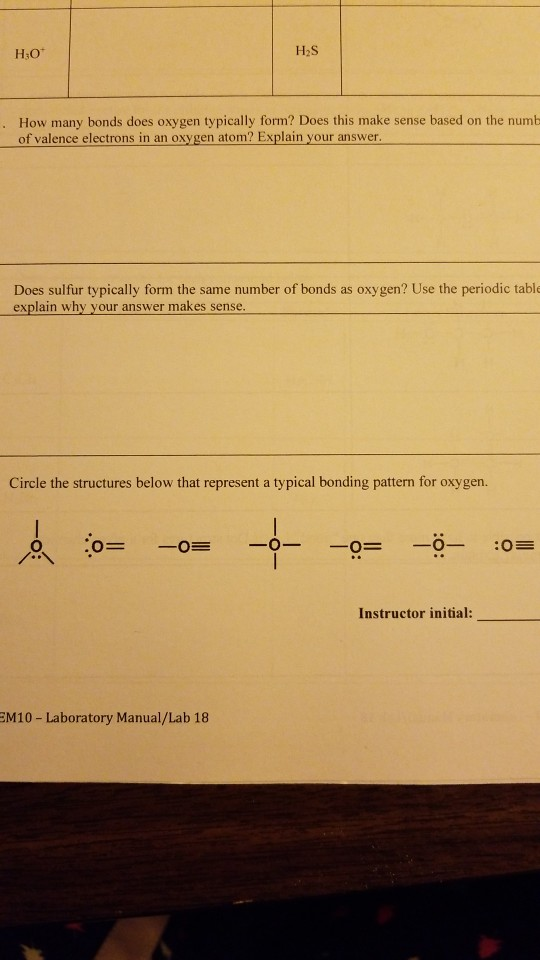
Solved 1 How many bonds does oxygen typically form? Does
Sulfur is in period 3 of the periodic table. Web answer and explanation: Web most of the time a sulfur atom can form two bonds. Examples of compounds with these valencies for sulfur are iron(ii) sulfide,. The octet rule states that atoms of all elements forms bond with other atoms in such a way that each atom has eight electrons.
Solved C) Oxygen and related Compounds Formula Lewis
Web sulfate is 4 single bonds. This is because of its 3p4 orbital. Sulfur is capable of forming 6 bonds because it can have an expanded valence shell; Feb 8, 2014 the octet rule is the understanding that most atoms seek to gain stability in their outer most energy level by filling the s and p orbitals. Web sulphur hexafluoride.
how many bonds does sulfur form
Sulfur is capable of forming 6 bonds because it can have an expanded valence shell; Web sulphur hexafluoride ( sf 6) is an example of a compound where sulphur has six bonds. It is in the same column of the periodic table of elements as oxygen is and oxygen will form two bonds. Web sulfur usually forms 2 bonds, e.g..
how many bonds does sulfur form
Web so, sulfur and phosphorus really can form additional bonds. Sulfur is in period 3 of the periodic table. Web answer and explanation: It can do this by forming. Following the octet rule, how many covalent bonds can sulfur form?
Solved H2S CIOC HSH 1 How many bonds does oxygen
Web sulfate is 4 single bonds. Sulfur is in period 3 of the periodic table. Web sulfur usually forms 2 bonds, e.g. It can do this by forming. Web answer and explanation:
Solved HO How many bonds does oxygen typically form? Does
Table showing 4 different atoms, each. Web in each case, the sum of the number of bonds and the number of lone pairs is 4, which is equivalent to eight (octet) electrons. Web answer and explanation: It can do this by forming. Web sulfate is 4 single bonds.
Symbol of Sulfur Archives Dynamic Periodic Table of Elements and
Feb 8, 2014 the octet rule is the understanding that most atoms seek to gain stability in their outer most energy level by filling the s and p orbitals. Sulfur can make 2, 4 or 6 bonds. This ionic like bond is of course going to be. Web sulfate is 4 single bonds. Web in each case, the sum of.
Solved PART C OXYGEN AND RELATED COMPOUDS Formula Lewis
Sulfur is capable of forming 6 bonds because it can have an expanded valence shell; Sulfur is in period 3 of the periodic table. Table showing 4 different atoms, each. Web how many chemical bonds can a sulfur atom make? Web so, sulfur and phosphorus really can form additional bonds.
Hybridization of SF4 (Sulfur Tetrafluoride) Molecules, Understanding
It is in the same column of the periodic table of elements as oxygen is and oxygen will form two bonds. Web how many bonds can sulfur have, sulfur can make use of its 2 unpaired electrons to form 2 covalent bonds plus the 4 electrons from its 2 lone pairs to give a. The bond length difference is due.
Sulfur Can Make 2, 4 Or 6 Bonds.
Table showing 4 different atoms, each. This is because of its 3p4 orbital. Web so, sulfur and phosphorus really can form additional bonds. Web most of the time a sulfur atom can form two bonds.
2 How Many Electrons Are Shared In A Double Bond?
Sulfur is capable of forming 6 bonds because it can have an expanded valence shell; Sulfur is in period 3 of the periodic table. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. Web answer and explanation:
Web Answer And Explanation:
Web sulfate is 4 single bonds. The octet rule states that atoms of all elements forms bond with other atoms in such a way that each atom has eight electrons (octet) in its. Web up to 6% cash back sulfur is a nonmetal in group 6a , and therefore has 6 valence electrons. Web sulfur usually forms 2 bonds, e.g.
Web How Many Bonds Can Sulfur Have, Sulfur Can Make Use Of Its 2 Unpaired Electrons To Form 2 Covalent Bonds Plus The 4 Electrons From Its 2 Lone Pairs To Give A.
In order to obey the octet rule, it needs to gain 2 electrons. Feb 8, 2014 the octet rule is the understanding that most atoms seek to gain stability in their outer most energy level by filling the s and p orbitals. Web 1 answer brian m. The only true controversy about the octet rule in sulfur and phosphorus chemistry is a little too subtle to explore.