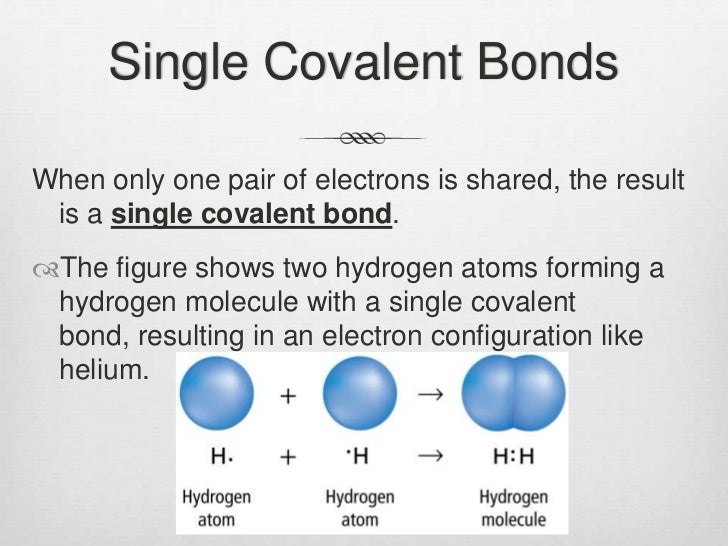
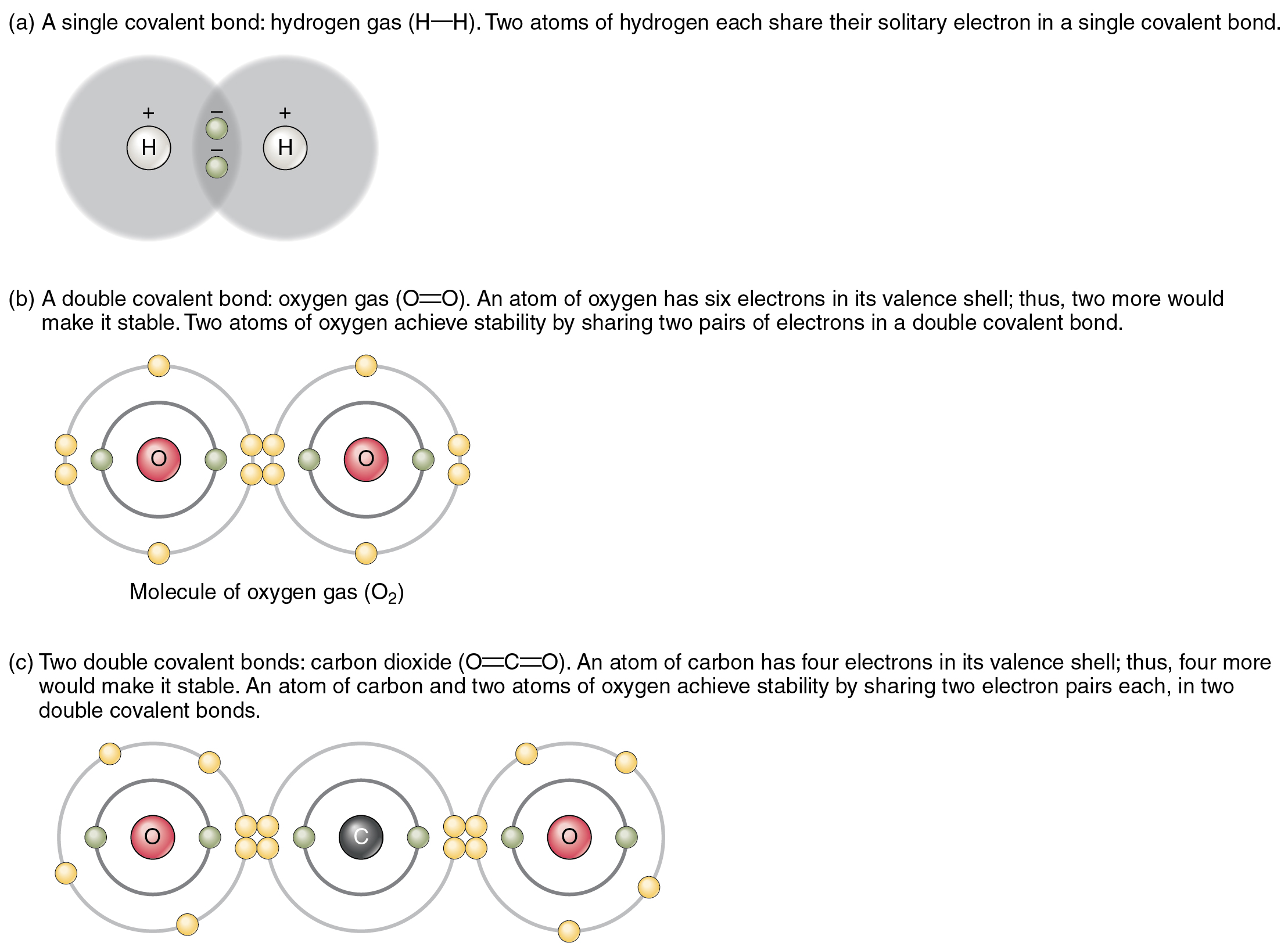
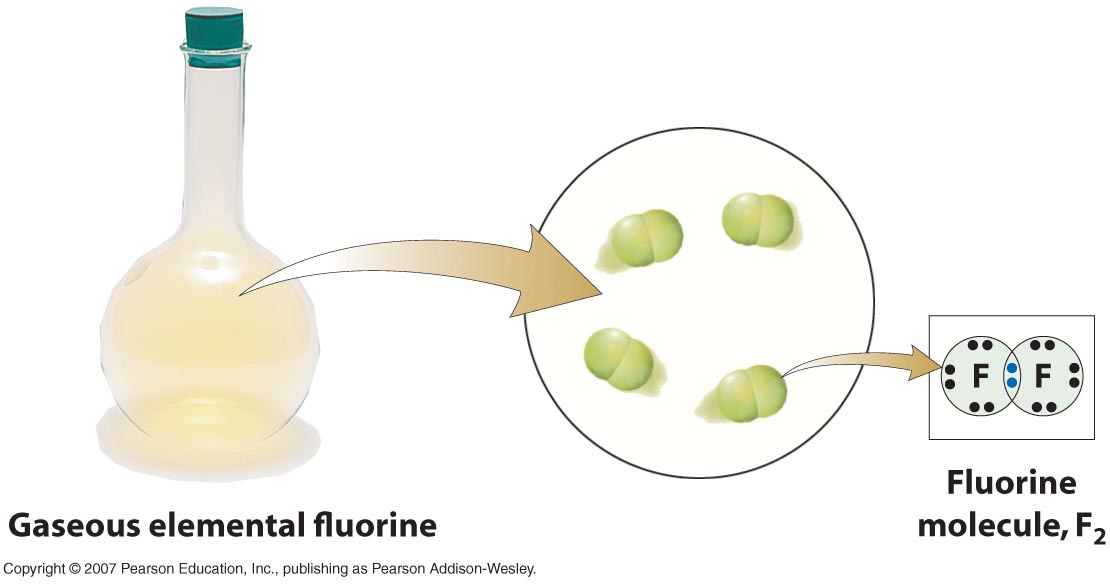
How Many Covalent Bonds Can Fluorine Form
How Many Covalent Bonds Can Fluorine Form - Web describe the formation of covalent bonds. Without performing any sophisticated analysis of its orbitals, we. Web like for dioxygen, three different states of this molecule are known: Two f's with two dots in. Web each fluorine atom contributes one valence electron, making a single bond and giving each atom a complete valence shell, which fulfills the octet rule: Create your account view this answer a fluorine atom (by itself) has 7 valence electrons. You can tell upon its formula. Ionic bonding results from the electrostatic attraction of oppositely. Why does fluorine have 3 lone. Web how many single covalent bonds can fluorine form?
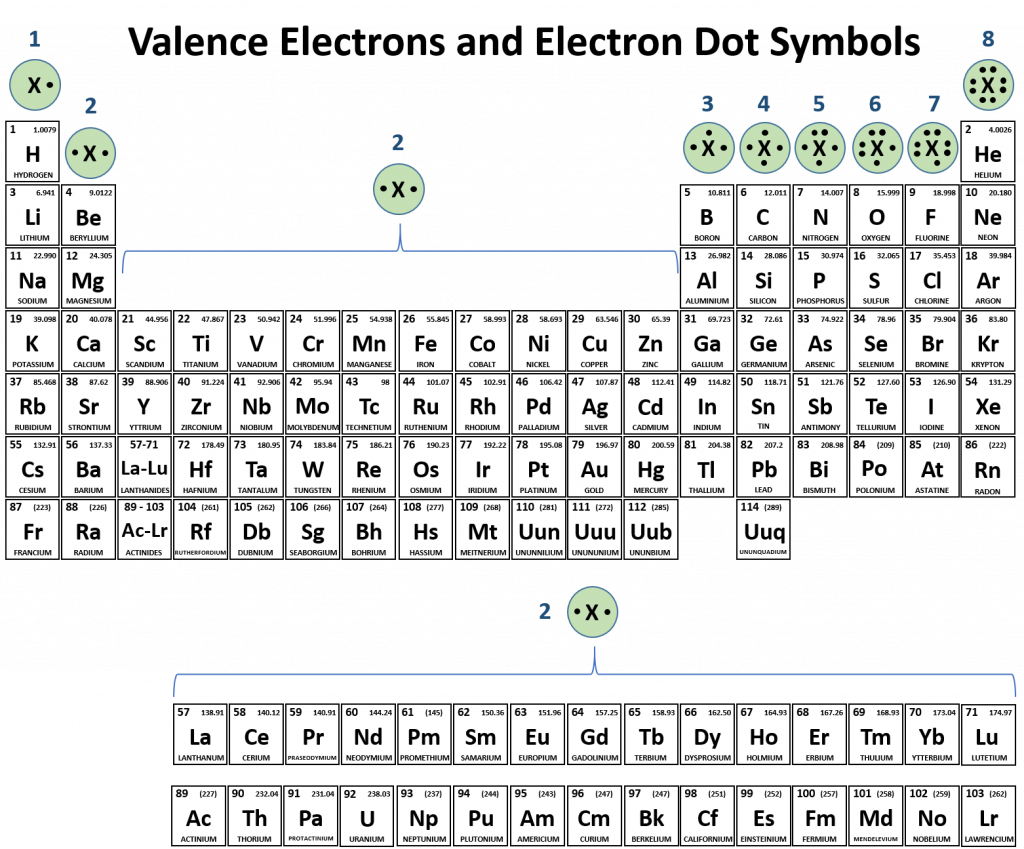
Web how many valence electrons are in a fluorine atom will fluorine atoms form bonds explain? You can tell upon its formula. One substance mentioned previously is wat (h₂o). Web how many covalent bonds does fluorine typically form? An atom of any halogen, such as fluorine, has seven valence electrons. Why does fluorine have 3 lone. The electrons involved are in. Fluorine will form covalent and. So it form 1 covalent bo. A) 1 b) 2 c) 3 d) 4 a which of the following ionic compounds has the largest lattice energy (i.e., the lattice energy most.
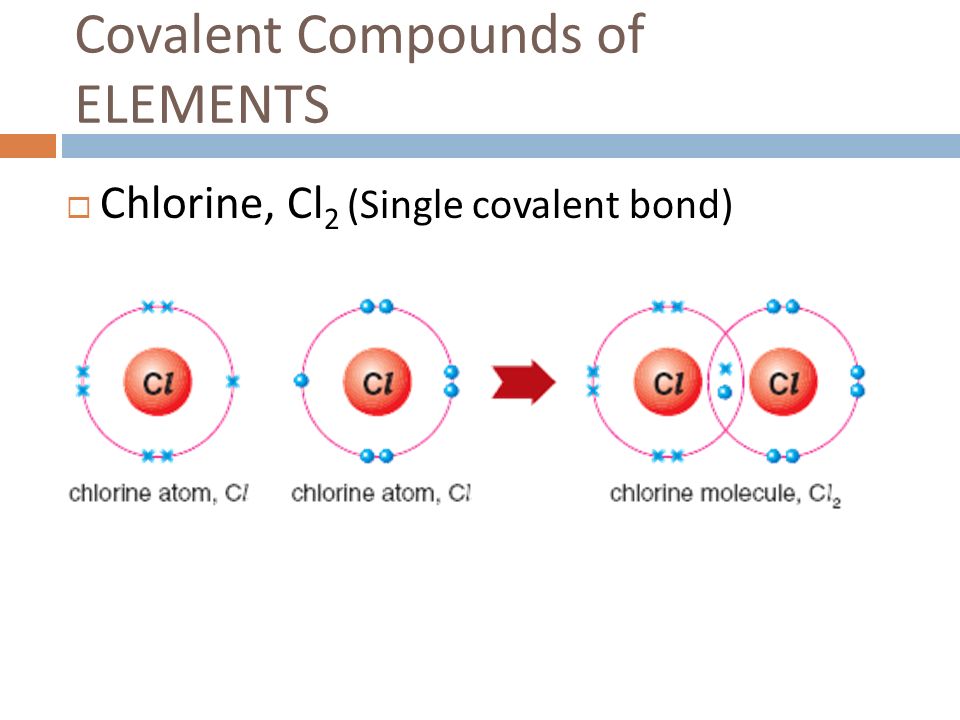
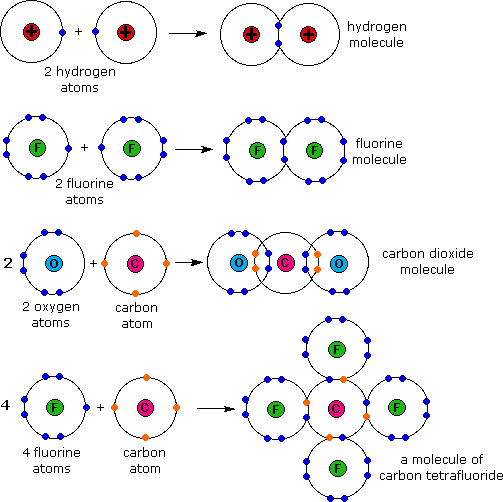
Web when two fluorine atoms come together, they each share one of their 7 valence electrons to form a nonpolar covalent bond. The high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character. Define electronegativity and assess the polarity of covalent bonds. Fluorine and the other halogens in group 7a (17) have seven valence electrons and can obtain an octet. Why does fluorine have 3 lone. Fluorine will form covalent and. Web how many covalent bonds does fluorine typically form? So it form 1 covalent bo. You'll get a detailed solution from a subject matter expert that helps. Web like for dioxygen, three different states of this molecule are known:
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Web how many covalent bonds does fluorine typically form? One substance mentioned previously is wat (h₂o). You'll get a detailed solution from a subject matter expert that helps. An atom of any halogen, such as fluorine, has seven valence electrons. Without performing any sophisticated analysis of its orbitals, we.
Chlorine combined with two negative atom or 1 positive and other
Create your account view this answer a fluorine atom (by itself) has 7 valence electrons. The electrons involved are in. You'll get a detailed solution from a subject matter expert that helps. Why does fluorine have 3 lone. Web how many single covalent bonds can fluorine form?
polarity Definition & Examples Britannica
Fluorine and the other halogens in group 7a (17) have seven valence electrons and can obtain an octet. Why does fluorine have 3 lone. Web how many covalent bonds does fluorine typically form? Define electronegativity and assess the polarity of covalent bonds. Without performing any sophisticated analysis of its orbitals, we.
__TOP__ How Many Covalent Bonds Can Chlorine Form
One triplet and two singlet states. Web how many covalent bonds can an atom of fluorine form? Two f's with two dots in. Web describe the formation of covalent bonds. Ionic bonding results from the electrostatic attraction of oppositely.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Web describe the formation of covalent bonds. Define electronegativity and assess the polarity of covalent bonds. Web each fluorine atom contributes one valence electron, making a single bond and giving each atom a complete valence shell, which fulfills the octet rule: Web when two fluorine atoms come together, they each share one of their 7 valence electrons to form a.
Atoms, Isotopes, Ions, and Molecules The Building Blocks · Biology
Web how many single covalent bonds can fluorine form? Web describe the formation of covalent bonds. Two f's with two dots in. Fluorine and the other halogens in group 7a (17) have seven valence electrons and can obtain an octet. Without performing any sophisticated analysis of its orbitals, we.
The Periodic Table and Bonding Mrs. Sanborn's Site
Without performing any sophisticated analysis of its orbitals, we. Two f's with two dots in. Web fluorine belongs to halogen family and has seven electrons in its valence shell, it can lose 7 electrons or gain 1 electron to attain stable configuration. Fluorine and the other halogens in group 7a (17) have seven valence electrons and can obtain an octet..
How many covalent bonds can hydrogen form?
The high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character. Fluorine and the other halogens in group 7a (17) have seven valence electrons and can obtain an octet. Web how many covalent bonds can an atom of fluorine form? Ionic bonding results from the electrostatic attraction of oppositely. One.
Chemical Bonds Anatomy & Physiology
A) 1 b) 2 c) 3 d) 4 a which of the following ionic compounds has the largest lattice energy (i.e., the lattice energy most. The electrons involved are in. You'll get a detailed solution from a subject matter expert that helps. Fluorine and the other halogens in group 7a (17) have seven valence electrons and can obtain an octet..
Why do two fluorine atoms bond together? Socratic
Define electronegativity and assess the polarity of covalent bonds. One substance mentioned previously is wat (h₂o). Web like for dioxygen, three different states of this molecule are known: Ionic bonding results from the electrostatic attraction of oppositely. You'll get a detailed solution from a subject matter expert that helps.
Without Performing Any Sophisticated Analysis Of Its Orbitals, We.
This problem has been solved! Web like for dioxygen, three different states of this molecule are known: Fluorine will form covalent and. Web fluorine belongs to halogen family and has seven electrons in its valence shell, it can lose 7 electrons or gain 1 electron to attain stable configuration.
1 Become A Study.com Member To Unlock This Answer!
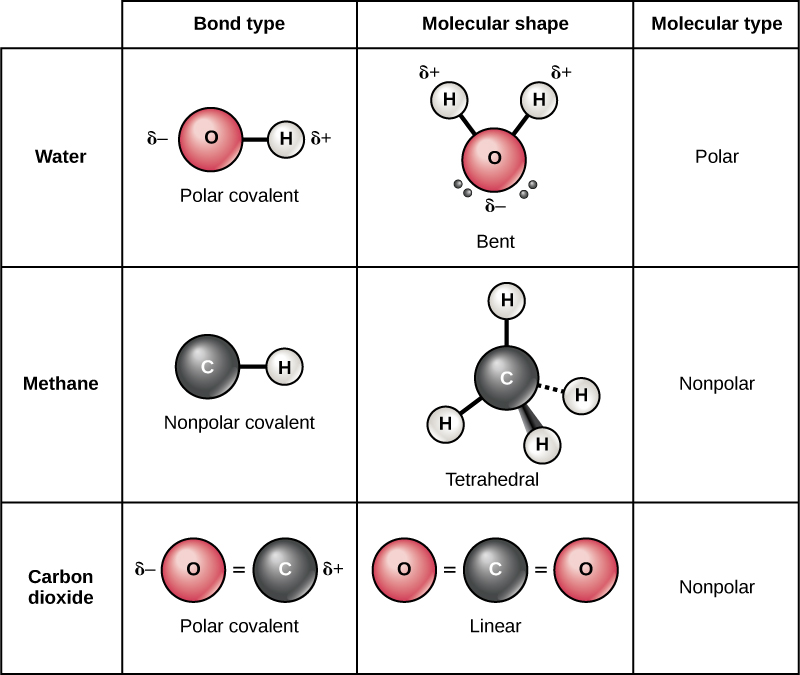
Web you have already seen examples of substances that contain covalent bonds. You can tell upon its formula. One triplet and two singlet states. Fluorine and the other halogens in group 7a (17) have seven valence electrons and can obtain an octet.
Web How Many Covalent Bonds Can An Atom Of Fluorine Form?
The high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character. Web how many single covalent bonds can fluorine form? So it form 1 covalent bo. Web how many covalent bonds does fluorine typically form?
Web The Halogens Also Form Single Covalent Bonds To Produce Diatomic Molecules.
Web each fluorine atom contributes one valence electron, making a single bond and giving each atom a complete valence shell, which fulfills the octet rule: Define electronegativity and assess the polarity of covalent bonds. Web how many valence electrons are in a fluorine atom will fluorine atoms form bonds explain? The polar nature of the bond.