How Many Covalent Bonds Can Phosphorus Form
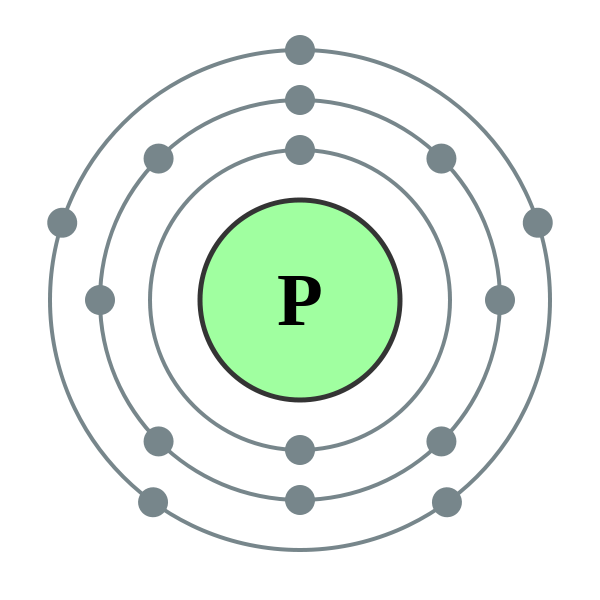
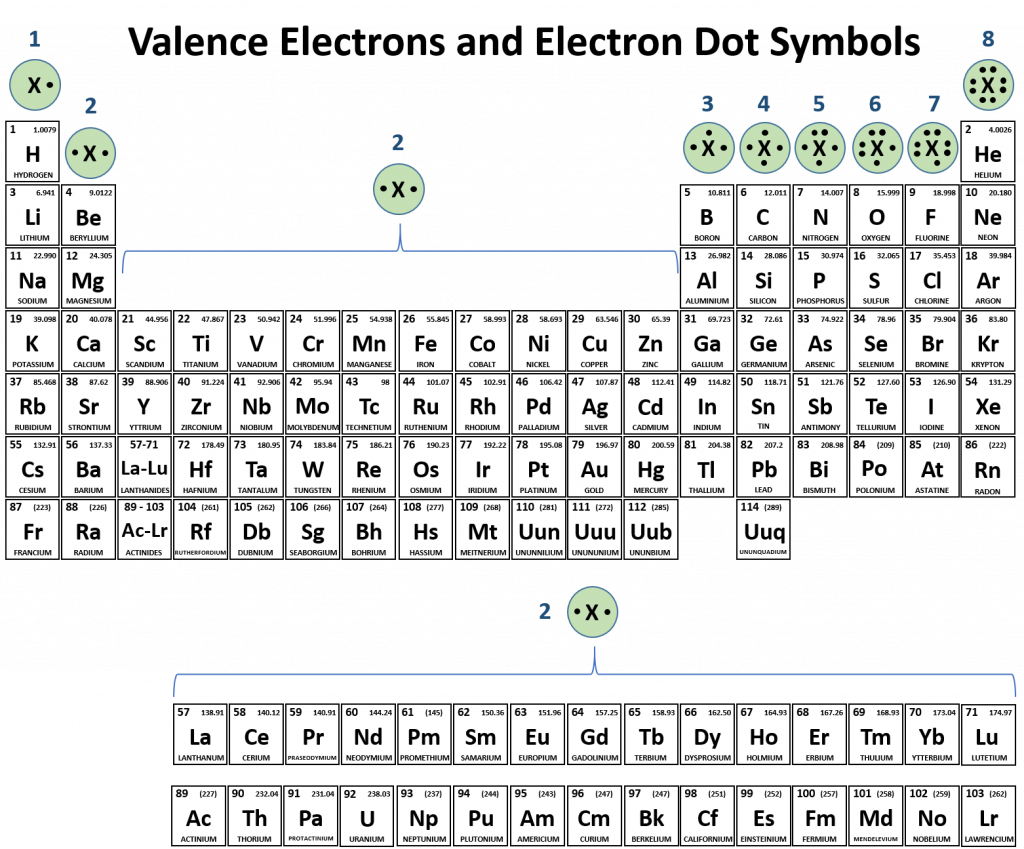
How Many Covalent Bonds Can Phosphorus Form - Phosphorous has 5 valence electrons like nitrogen and has a good chance of forming 3 bonds with one lone pair to make an octet. When it is large, the bond is polar covalent or ionic. But phosphorous has empty d. Two combinations of atoms can produce this type of bonding: Web phosphorous pentachloride shares five pairs of electrons for a total of ten electrons in the valence shell. Web typically, the atoms of group 4a form 4 covalent bonds; Web how many bonds does phosphorus typically make? Web when the difference is very small or zero, the bond is covalent and nonpolar. Correct option is b) phosphorous has an atomic number of 15 and electronic configuration as 1s 22s 22p 63s 23p 3. Covalent bond two atoms form a covalent chemical bond by each sharing at least one of their available valence.
Web this type of bonding would be a covalent bond. When phosphorus burns in chlorine both are. Web how many bonds does phosphorus typically make? Web phosphorus doesn’t need to follow the octet rule. Correct option is b) phosphorous has an atomic number of 15 and electronic configuration as 1s 22s 22p 63s 23p 3. In some expanded octet molecules, such as if 5 and xef. Web in the pcl3 molecule, each chlorine is bonded by a single covalent bond with phosphorus. Web how many covalent bonds is phosphorus? Group 5a form 3 bonds; Web phosphorous pentachloride shares five pairs of electrons for a total of ten electrons in the valence shell.
So there are three covalent bonds and one lone pair of electrons on. Web in the pcl3 molecule, each chlorine is bonded by a single covalent bond with phosphorus. Web the number of valence electrons in phosphorus is generally 5 and it requires 3 more electrons to complete its octet. Web this type of bonding would be a covalent bond. Therefore phosphorus maximum covalency of 6. When phosphorus burns in chlorine both are. Web when the difference is very small or zero, the bond is covalent and nonpolar. Group 6a form 2 bonds; Two combinations of atoms can produce this type of bonding: Web how many bonds does phosphorus typically make?
Atoms, Isotopes, Ions, and Molecules The Building Blocks · Biology
Two combinations of atoms can produce this type of bonding: Group 6a form 2 bonds; Phosphorous has 5 valence electrons like nitrogen and has a good chance of forming 3 bonds with one lone pair to make an octet. Web typically, the atoms of group 4a form 4 covalent bonds; Web answer (1 of 3):
Atomic Thoery Timeline
Web when the difference is very small or zero, the bond is covalent and nonpolar. Web phosphorous pentachloride shares five pairs of electrons for a total of ten electrons in the valence shell. Web how many covalent bonds is phosphorus? Web typically, the atoms of group 4a form 4 covalent bonds; When phosphorus burns in chlorine both are.
Forming covalent compounds CheMystery
In some expanded octet molecules, such as if 5 and xef. Web the number of valence electrons in phosphorus is generally 5 and it requires 3 more electrons to complete its octet. Web phosphorous pentachloride shares five pairs of electrons for a total of ten electrons in the valence shell. Web this type of bonding would be a covalent bond..
How many covalent bonds can hydrogen form?
Phosphorous has 5 valence electrons like nitrogen and has a good chance of forming 3 bonds with one lone pair to make an octet. When it is large, the bond is polar covalent or ionic. Web there are many different modifications of phosphorus in nature. When phosphorus burns in chlorine both are. Web phosphorous pentachloride shares five pairs of electrons.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
In some expanded octet molecules, such as if 5 and xef. Web there are many different modifications of phosphorus in nature. Web how many covalent bonds is phosphorus? Web how many bonds does phosphorus typically make? Web answer (1 of 3):
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Web when the difference is very small or zero, the bond is covalent and nonpolar. Web how many bonds does phosphorus typically make? In some expanded octet molecules, such as if 5 and xef. Two combinations of atoms can produce this type of bonding: So, phosphorous can exceed its.
__TOP__ How Many Covalent Bonds Can Chlorine Form
Group 6a form 2 bonds; But phosphorous has empty d. Web this type of bonding would be a covalent bond. So, phosphorous can exceed its. Correct option is b) phosphorous has an atomic number of 15 and electronic configuration as 1s 22s 22p 63s 23p 3.
polarity Definition & Examples Britannica
Unlike nitrogen phosphorus has 2 vacant d. When phosphorus burns in chlorine both are. Web how many bonds does phosphorus typically make? Web this type of bonding would be a covalent bond. Correct option is b) phosphorous has an atomic number of 15 and electronic configuration as 1s 22s 22p 63s 23p 3.
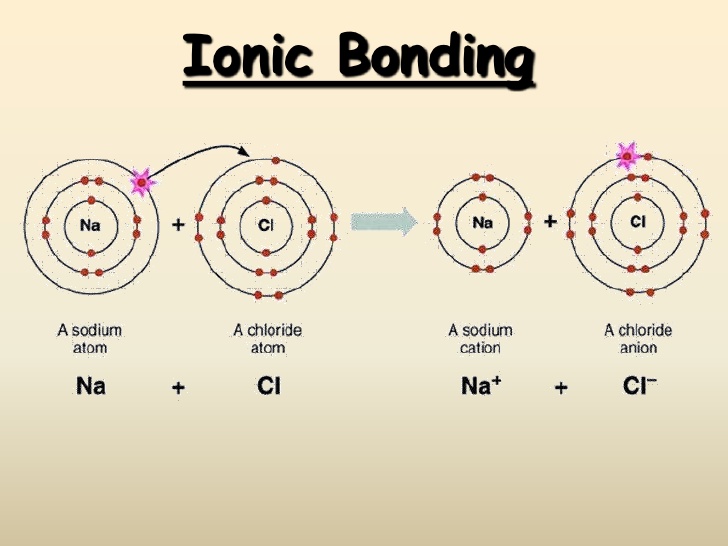
What are similarities of covalent and ionic bonding? Socratic
Web typically, the atoms of group 4a form 4 covalent bonds; Web phosphorus doesn’t need to follow the octet rule. Web the number of valence electrons in phosphorus is generally 5 and it requires 3 more electrons to complete its octet. The absolute values of the electronegativity. In some expanded octet molecules, such as if 5 and xef.
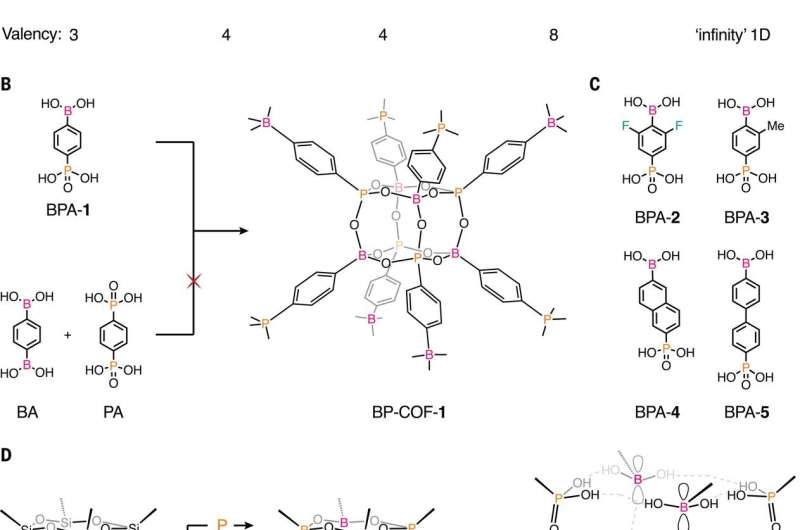
New covalent organic framework using boron and phosphorus allows for
Web when the difference is very small or zero, the bond is covalent and nonpolar. Web typically, the atoms of group 4a form 4 covalent bonds; In some expanded octet molecules, such as if 5 and xef. So there are three covalent bonds and one lone pair of electrons on. And group 7a form one bond.
So There Are Three Covalent Bonds And One Lone Pair Of Electrons On.
Two combinations of atoms can produce this type of bonding: Group 6a form 2 bonds; Correct option is b) phosphorous has an atomic number of 15 and electronic configuration as 1s 22s 22p 63s 23p 3. Web in the pcl3 molecule, each chlorine is bonded by a single covalent bond with phosphorus.
In Some Expanded Octet Molecules, Such As If 5 And Xef.
Web the number of valence electrons in phosphorus is generally 5 and it requires 3 more electrons to complete its octet. And group 7a form one bond. Web typically, the atoms of group 4a form 4 covalent bonds; But phosphorous has empty d.
So, Phosphorous Can Exceed Its.
Web at most, how many covalent bonds can phosphorus atom form? Covalent bond two atoms form a covalent chemical bond by each sharing at least one of their available valence. Group 5a form 3 bonds; Web how many covalent bonds is phosphorus?
Therefore Phosphorus Maximum Covalency Of 6.
The absolute values of the electronegativity. Web answer (1 of 3): Unlike nitrogen phosphorus has 2 vacant d. Web phosphorous pentachloride shares five pairs of electrons for a total of ten electrons in the valence shell.