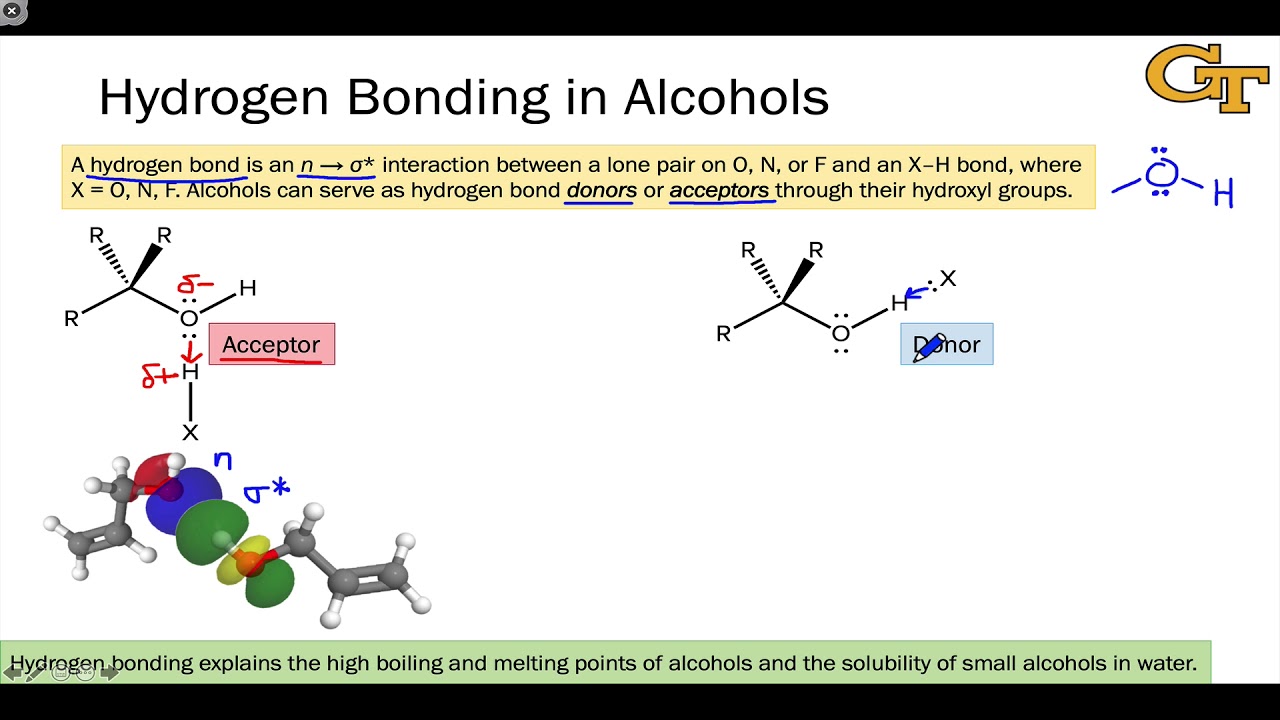
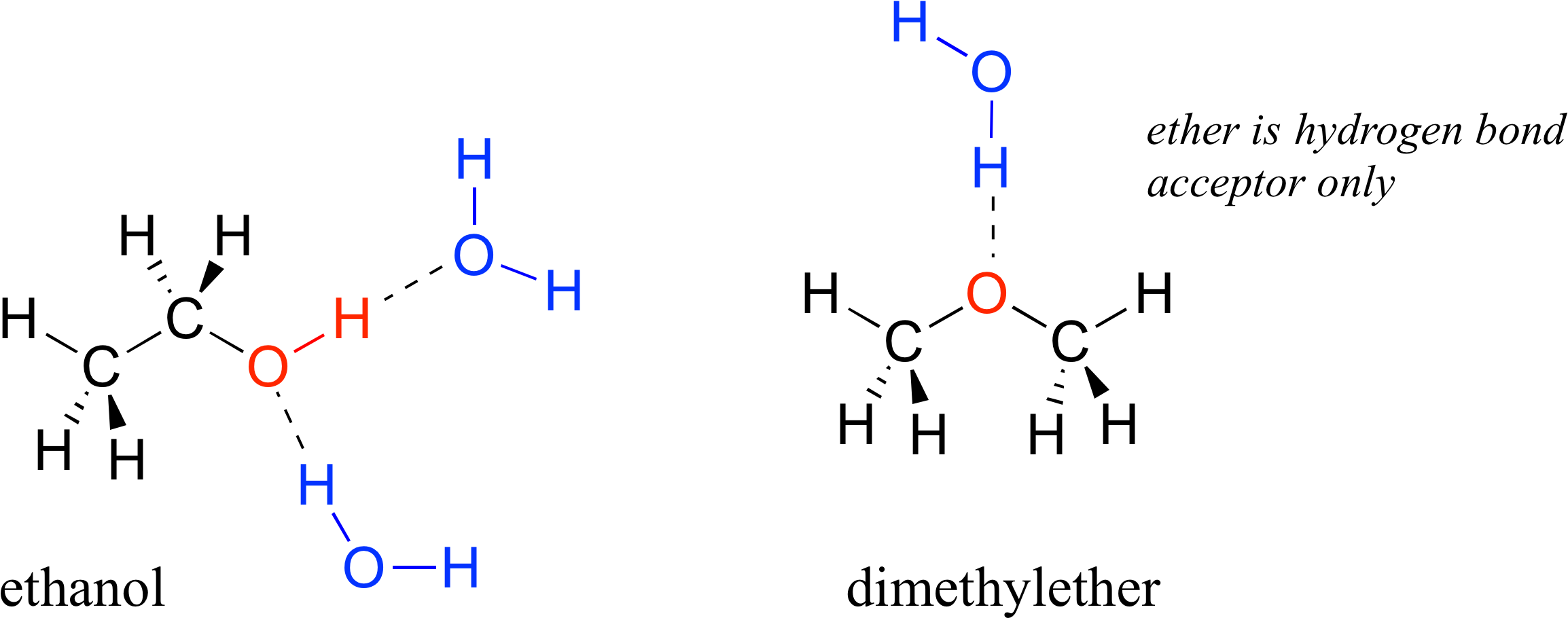
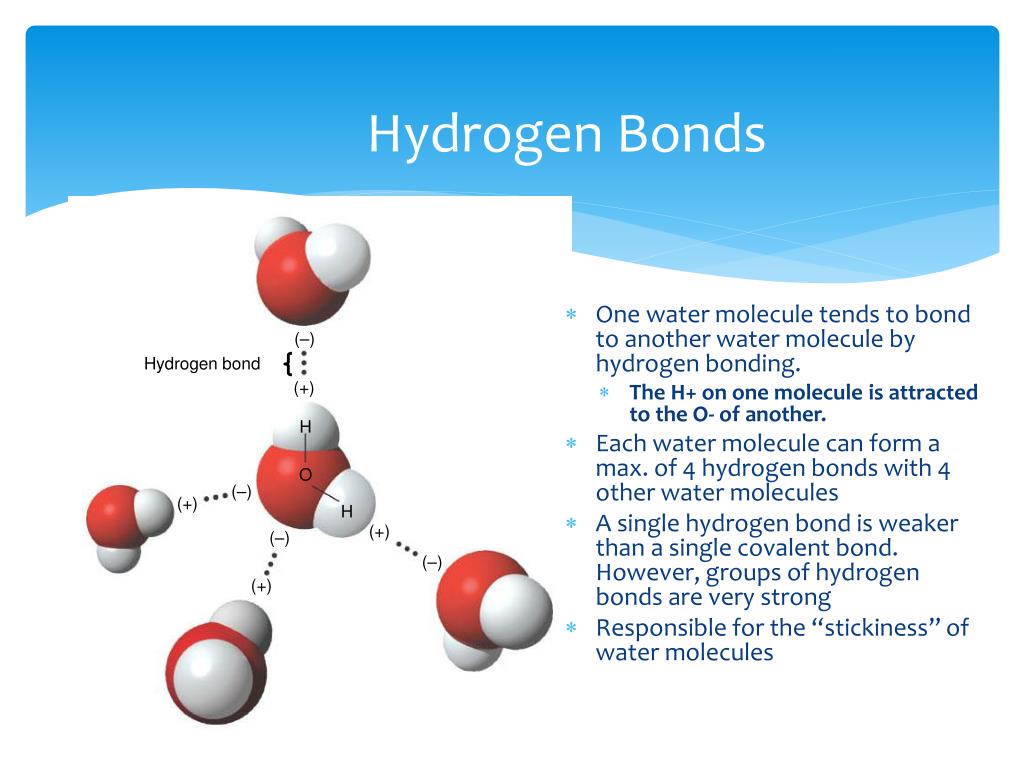
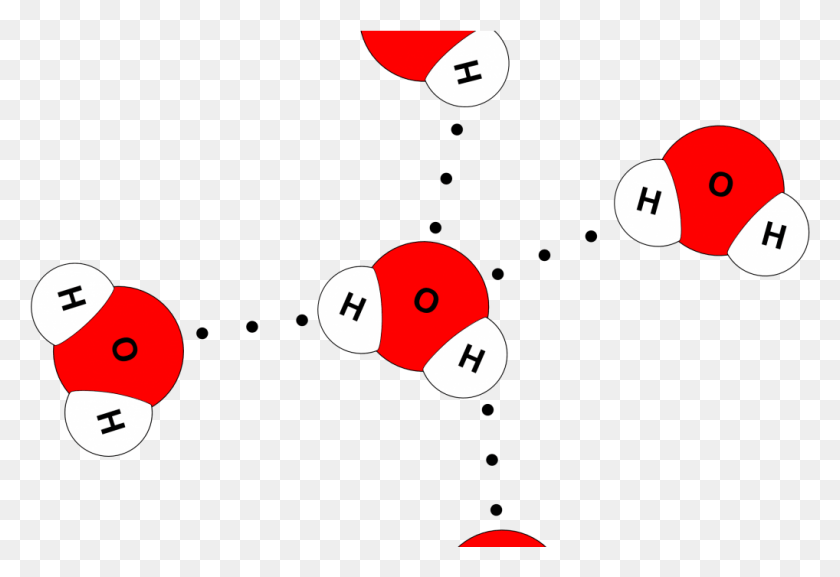
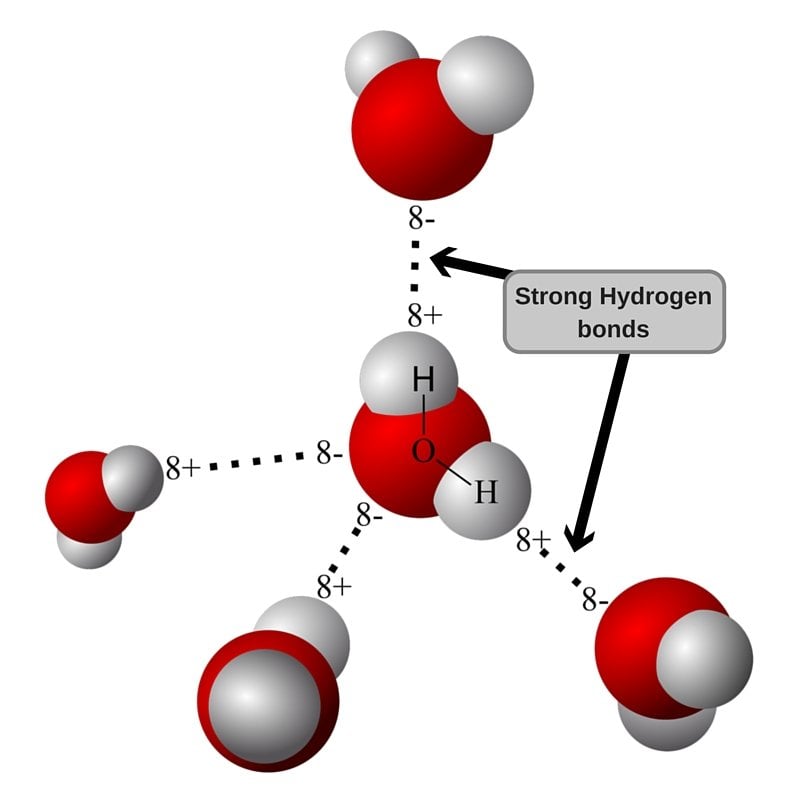
How Many Hydrogen Bonds Can Water Form
How Many Hydrogen Bonds Can Water Form - Web the 4 possible hydrogen bonds formed with a water molecule in ice. Web water is can ideally model of hydrogen stick. Web a molecule of water has two hydrogen atoms. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Web in the liquid phase, the average number of hydrogen bonds that a water molecule participates in varies greatly with temperature, the average being 3.69 at 0ºc,. There are exactly the right numbers of + hydrogens. Notice that everyone water molecule can potentially form four hydrogen debenture the surrounds aquarium molecules: Both an oxygen atom and 2 hydrogen atoms in one molecule. 1 answer evan holbrook jun 21, 2018 due to the large difference in electronegativity between oxygen. Web why does water form hydrogen bonds?
Web in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Every water molecule can be. Web in the liquid phase, the average number of hydrogen bonds that a water molecule participates in varies greatly with temperature, the average being 3.69 at 0ºc,. Web up to 4 hydrogen bonds can form between a single water molecule and other water molecules. 1 answer evan holbrook jun 21, 2018 due to the large difference in electronegativity between oxygen. Notice that everyone water molecule can potentially form four hydrogen debenture the surrounds aquarium molecules: The number of hydrogen bonds formed/molecule in liquid water is less than four, and decreases as the. Water (h 2 o) is a simple triatomic bent molecule with c 2v molecular symmetry and bond angle of 104.5°. It makes two with the. Both an oxygen atom and 2 hydrogen atoms in one molecule.
The two lone pairs of oxygen atoms and the two hydrogen atoms of water are involved in intermolecular hydrogen bonding. Web answer (1 of 15): Web in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. In h 2 o, only two of the six. Water (h 2 o) is a simple triatomic bent molecule with c 2v molecular symmetry and bond angle of 104.5°. Web why does water form hydrogen bonds? It makes two with the. There are exactly the right numbers of + hydrogens. Web water is can ideally model of hydrogen stick. Notice that everyone water molecule can potentially form four hydrogen debenture the surrounds aquarium molecules:
Difference Between Intermolecular and Intramolecular Hydrogen Bonding
In h 2 o, only two of the six. Water (h 2 o) is a simple triatomic bent molecule with c 2v molecular symmetry and bond angle of 104.5°. The number of hydrogen bonds formed/molecule in liquid water is less than four, and decreases as the. Every water molecule can be. Web up to 4 hydrogen bonds can form between.
Water
Notice that everyone water molecule can potentially form four hydrogen debenture the surrounds aquarium molecules: Every water molecule can be. Web water can form four hydrogen bonds. There are exactly the right numbers of + hydrogens. Web in the liquid phase, the average number of hydrogen bonds that a water molecule participates in varies greatly with temperature, the average being.
How many hydrogen bonds a water molecule can form Hydrogen Bonding in
Web water can form four hydrogen bonds. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. 1 answer evan holbrook jun 21, 2018 due to the large difference in electronegativity between oxygen. In h 2 o, only two of the six. Every water molecule can be.
How Many Hydrogen Bonds Can Ethanol Form Printable Form, Templates
Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. It makes two with the. Both of these atoms can form a hydrogen bond with oxygen atoms of different water molecules. In h 2 o, only two of the six. Both an oxygen atom and 2 hydrogen atoms in one molecule.
How many hydrogen bonds are attached to each water molecule in a solid
Web in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. There are exactly the right numbers of + hydrogens. Web up to 4 hydrogen bonds can form between a single water molecule and other water molecules. The two lone pairs of oxygen atoms and the two.
Download How Many Hydrogen Bonds Can A Water Molecule Form Hydrogen
The two lone pairs of oxygen atoms and the two hydrogen atoms of water are involved in intermolecular hydrogen bonding. It makes two with the. The number of hydrogen bonds formed/molecule in liquid water is less than four, and decreases as the. In h 2 o, only two of the six. Notice that everyone water molecule can potentially form four.
PPT Properties of Water PowerPoint Presentation, free download ID
It makes two with the. The two lone pairs of oxygen atoms and the two hydrogen atoms of water are involved in intermolecular hydrogen bonding. Both of these atoms can form a hydrogen bond with oxygen atoms of different water molecules. There are exactly the right numbers of + hydrogens. The number of hydrogen bonds formed/molecule in liquid water is.
Hexanol Model Molecule Carbon 3d Ball Stick Volatile Organic Compounds
Both an oxygen atom and 2 hydrogen atoms in one molecule. Web water is unique because its oxygen atom has two lone pairs and two hydrogen atoms, meaning that the total number of bonds of a water molecule is up to four. 1 answer evan holbrook jun 21, 2018 due to the large difference in electronegativity between oxygen. The number.
Why Do Fingers/Hands Stick To Ice? » Science ABC
Web lewis structure of h 2 o indicating bond angle and bond length. Web answer (1 of 15): Web in the liquid phase, the average number of hydrogen bonds that a water molecule participates in varies greatly with temperature, the average being 3.69 at 0ºc,. Web water is unique because its oxygen atom has two lone pairs and two hydrogen.
Bonds That Hold Water Molecules Together / Intermolecular Forces
Web the 4 possible hydrogen bonds formed with a water molecule in ice. It makes two with the. There are exactly the right numbers of + hydrogens. 1 answer evan holbrook jun 21, 2018 due to the large difference in electronegativity between oxygen. Water (h 2 o) is a simple triatomic bent molecule with c 2v molecular symmetry and bond.
Web In Water, Each Hydrogen Nucleus Is Covalently Bound To The Central Oxygen Atom By A Pair Of Electrons That Are Shared Between Them.
Both of these atoms can form a hydrogen bond with oxygen atoms of different water molecules. Water (h 2 o) is a simple triatomic bent molecule with c 2v molecular symmetry and bond angle of 104.5°. Web water is unique because its oxygen atom has two lone pairs and two hydrogen atoms, meaning that the total number of bonds of a water molecule is up to four. It makes two with the.
Web Water Is Can Ideally Model Of Hydrogen Stick.
The two lone pairs of oxygen atoms and the two hydrogen atoms of water are involved in intermolecular hydrogen bonding. In h 2 o, only two of the six. Web water can form four hydrogen bonds. Every water molecule can be.
There Are Exactly The Right Numbers Of + Hydrogens.
Notice that everyone water molecule can potentially form four hydrogen debenture the surrounds aquarium molecules: Both an oxygen atom and 2 hydrogen atoms in one molecule. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Web why does water form hydrogen bonds?
Web Answer (1 Of 15):
Web a molecule of water has two hydrogen atoms. Web up to 4 hydrogen bonds can form between a single water molecule and other water molecules. Web in the liquid phase, the average number of hydrogen bonds that a water molecule participates in varies greatly with temperature, the average being 3.69 at 0ºc,. 1 answer evan holbrook jun 21, 2018 due to the large difference in electronegativity between oxygen.