Identify The Keto Form Of Each Enol Tautomer
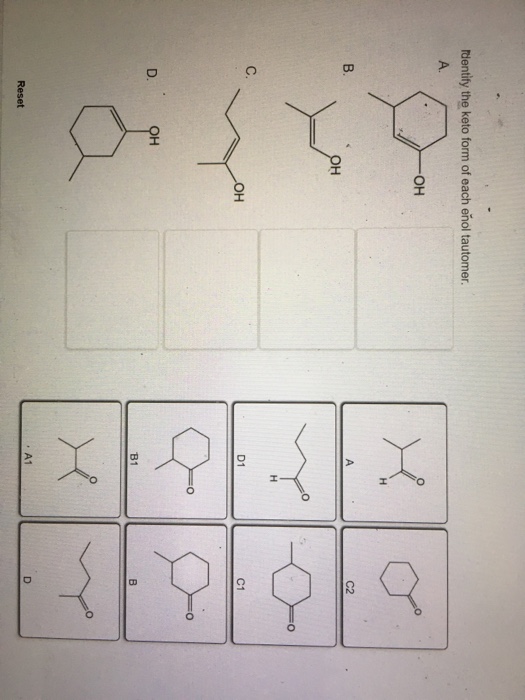
Identify The Keto Form Of Each Enol Tautomer - Solution verified answered this week answered this week step 1 1 of 6 enols can be formed in. 0.50 points identify the enol form of each keto tautomer. D1 a1 он oh b1 reset a. Web different combinations of enol to keto ratio. For simple carbonyl compounds under normal. Choose among the given choices. This problem has been solved! Он в1 d о н с1 а reset. You'll get a detailed solution from a subject matter expert that helps you. Note that each peak integration area is weighted according to the number of protons that contribute to the respective signal.
This process is catalyzed by both acid and a base. However, the keto form occasionally changes spontaneously to the. Note that each peak integration area is weighted according to the number of protons that contribute to the respective signal. Tautomers are rapidly interconverted constitutional. This problem has been solved! Он в1 d о н с1 а reset. For simple carbonyl compounds under normal. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Choose among the given choices. 0.50 points identify the enol form of each keto tautomer.
Web identify the enol form of each keto tautomer: For simple carbonyl compounds under normal. Solution verified answered this week answered this week step 1 1 of 6 enols can be formed in. However, the keto form occasionally changes spontaneously to the. Note that each peak integration area is weighted according to the number of protons that contribute to the respective signal. Protonation of enolate into oxygen leads to enol which is an unstable isomer of aldehyde or ketone and it quickly transforms into a carbonyl. D1 a1 он oh b1 reset a. This process is catalyzed by both acid and a base. 0.50 points identify the enol form of each keto tautomer. Choose among the given choices.
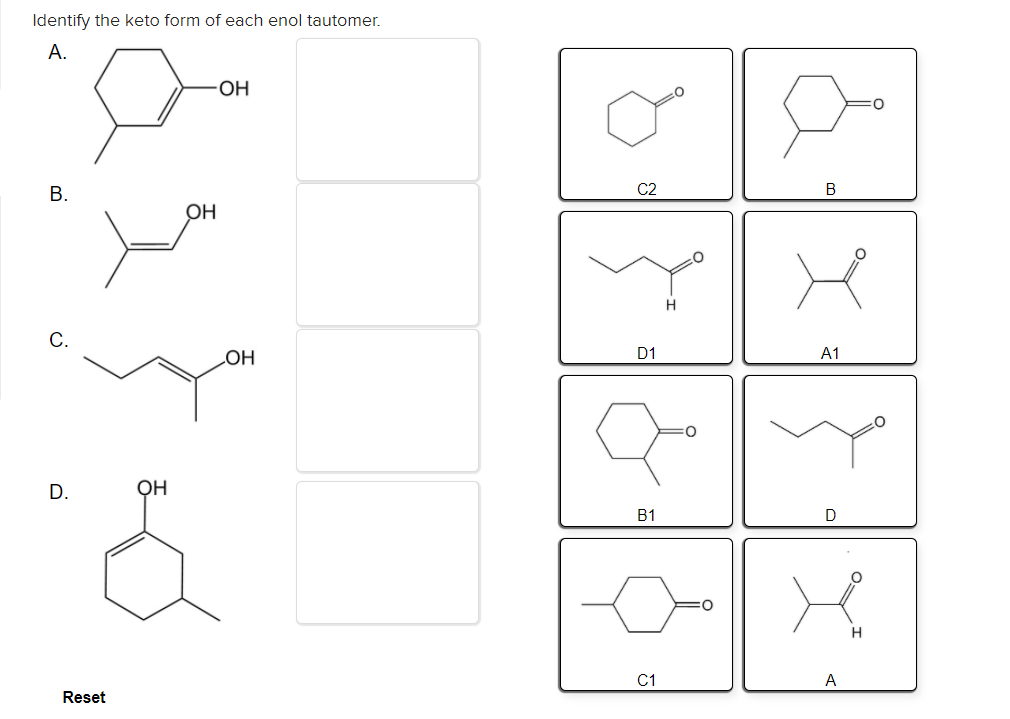
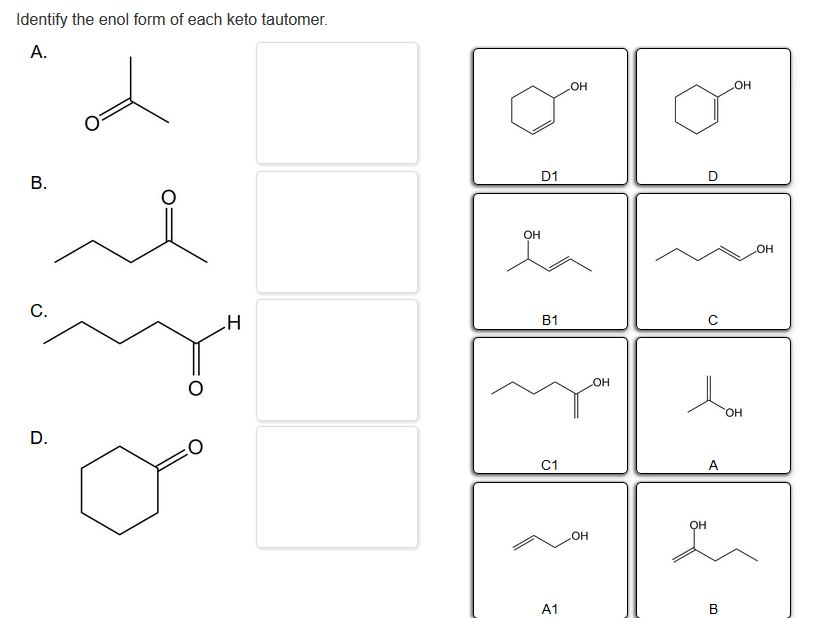
Solved Identify the keto form of each enol tautomer. А. ОН
Note that each peak integration area is weighted according to the number of protons that contribute to the respective signal. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web identify the enol form of each keto tautomer: However, the keto form occasionally changes spontaneously to the. D1 a1 он oh b1 reset.
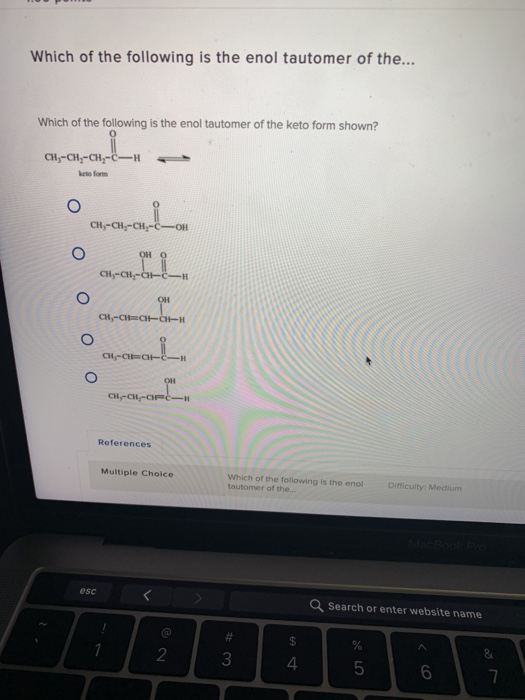
Solved Which of the following is the enol tautomer of the...
Choose among the given choices. You'll get a detailed solution from a subject matter expert that helps you. This problem has been solved! Tautomers are rapidly interconverted constitutional. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
Solved Select the keyword or phrase that will best complete
Web different combinations of enol to keto ratio. Web ldentify the enol form of each keto tautomer a. D1 a1 он oh b1 reset show. Identify the keto form of each enol tautomer. However, the keto form occasionally changes spontaneously to the.
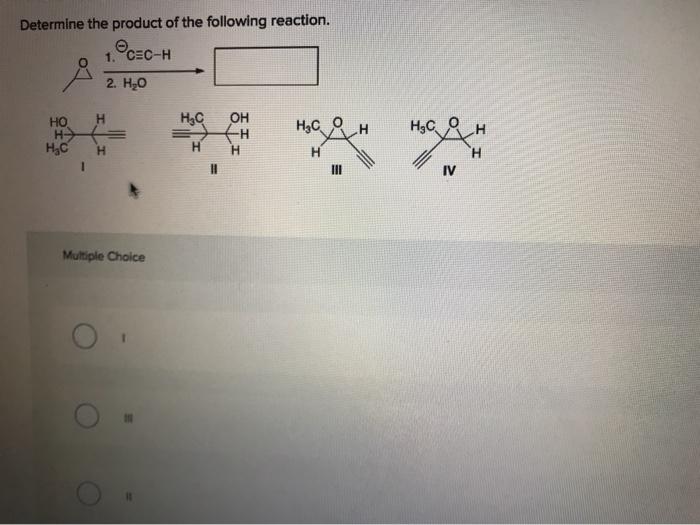
Solved Determine the product of the following reaction.
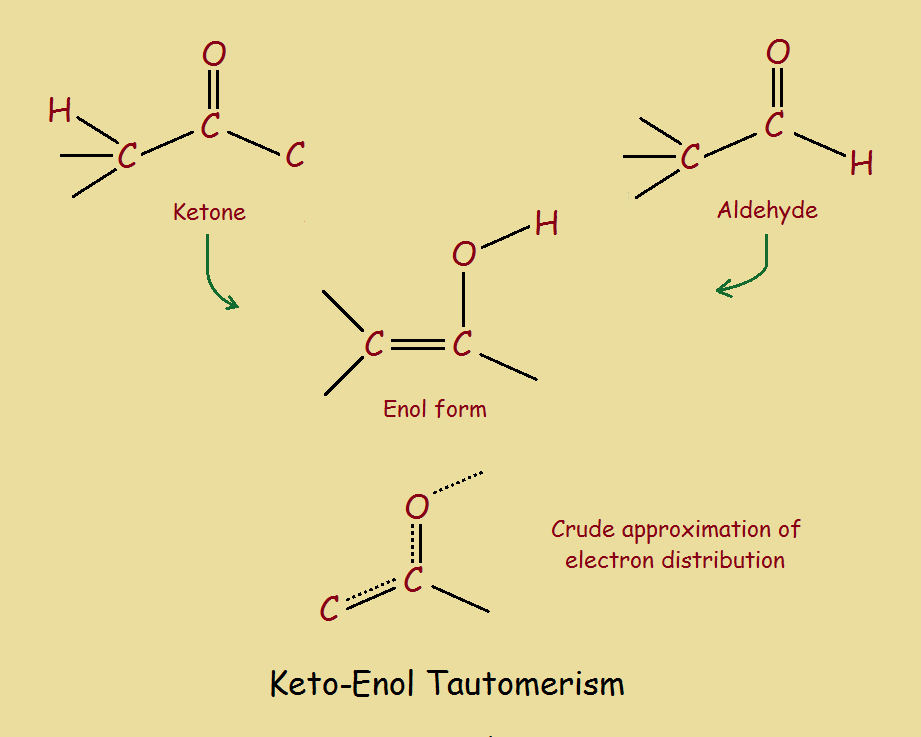
Protonation of enolate into oxygen leads to enol which is an unstable isomer of aldehyde or ketone and it quickly transforms into a carbonyl. Web identify the enol form of each keto tautomer. This process is catalyzed by both acid and a base. Он в1 d о н с1 а reset. However, the keto form occasionally changes spontaneously to the.
organic chemistry Which is the more stable enol form? Chemistry
For simple carbonyl compounds under normal. D1 a1 он oh b1 reset a. Web identify the enol form of each keto tautomer: Web ldentify the enol form of each keto tautomer a. Tautomers are rapidly interconverted constitutional.
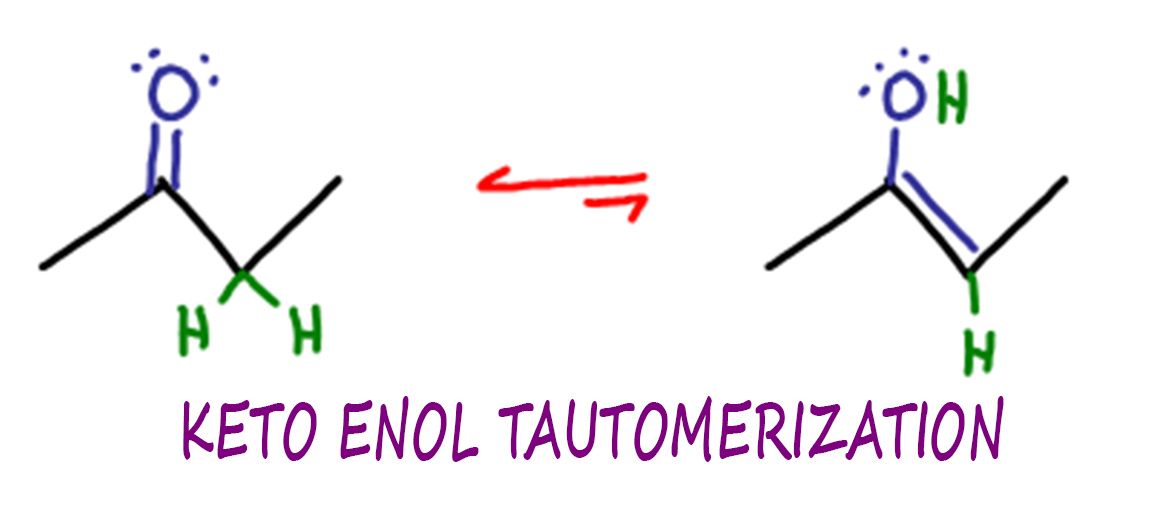
Keto Enol Tautomerization Reaction and Mechanism in Acid and Base
For simple carbonyl compounds under normal. Protonation of enolate into oxygen leads to enol which is an unstable isomer of aldehyde or ketone and it quickly transforms into a carbonyl. Tautomers are rapidly interconverted constitutional. 0.50 points identify the enol form of each keto tautomer. However, the keto form occasionally changes spontaneously to the.
Solved dentify the keto form of each enol tautomer он C2 C.
For simple carbonyl compounds under normal. A1 this problem has been solved! D1 a1 он oh b1 reset a. This problem has been solved! Он в1 d о н с1 а reset.
Keto Enol Tautomerism What Is It and Why Is It Important?
Identify the keto form of each enol tautomer. For simple carbonyl compounds under normal. Web identify the enol form of each keto tautomer: D1 a1 он oh b1 reset a. Tautomers are rapidly interconverted constitutional.
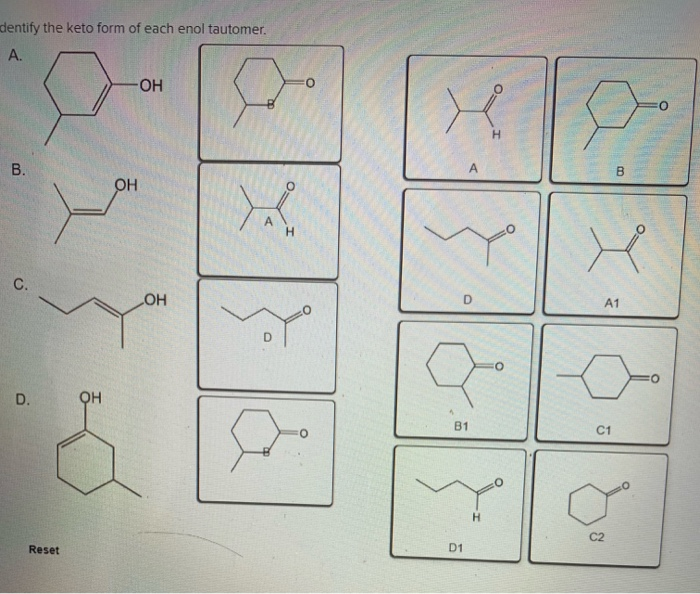
Solved Identify the enol form of each keto tautomer.
Identify the keto form of each enol tautomer. 0.50 points identify the enol form of each keto tautomer. However, the keto form occasionally changes spontaneously to the. A1 this problem has been solved! Web ldentify the enol form of each keto tautomer a.
KetoEnol Tautomerism Key Points Master Organic Chemistry
D1 a1 он oh b1 reset show. Web ldentify the enol form of each keto tautomer a. Solution verified answered this week answered this week step 1 1 of 6 enols can be formed in. A1 this problem has been solved! For simple carbonyl compounds under normal.
Note That Each Peak Integration Area Is Weighted According To The Number Of Protons That Contribute To The Respective Signal.
Choose among the given choices. For simple carbonyl compounds under normal. However, the keto form occasionally changes spontaneously to the. Web identify the enol form of each keto tautomer:
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You.
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. This problem has been solved! Identify the keto form of each enol tautomer. Он в1 d о н с1 а reset.
0.50 Points Identify The Enol Form Of Each Keto Tautomer.
Web different combinations of enol to keto ratio. Solution verified answered this week answered this week step 1 1 of 6 enols can be formed in. This process is catalyzed by both acid and a base. Web identify the enol form of each keto tautomer.
Web Ldentify The Enol Form Of Each Keto Tautomer A.
A1 this problem has been solved! Protonation of enolate into oxygen leads to enol which is an unstable isomer of aldehyde or ketone and it quickly transforms into a carbonyl. D1 a1 он oh b1 reset a. D1 a1 он oh b1 reset show.