In What Form Can An Ionic Compound Conduct Electricity
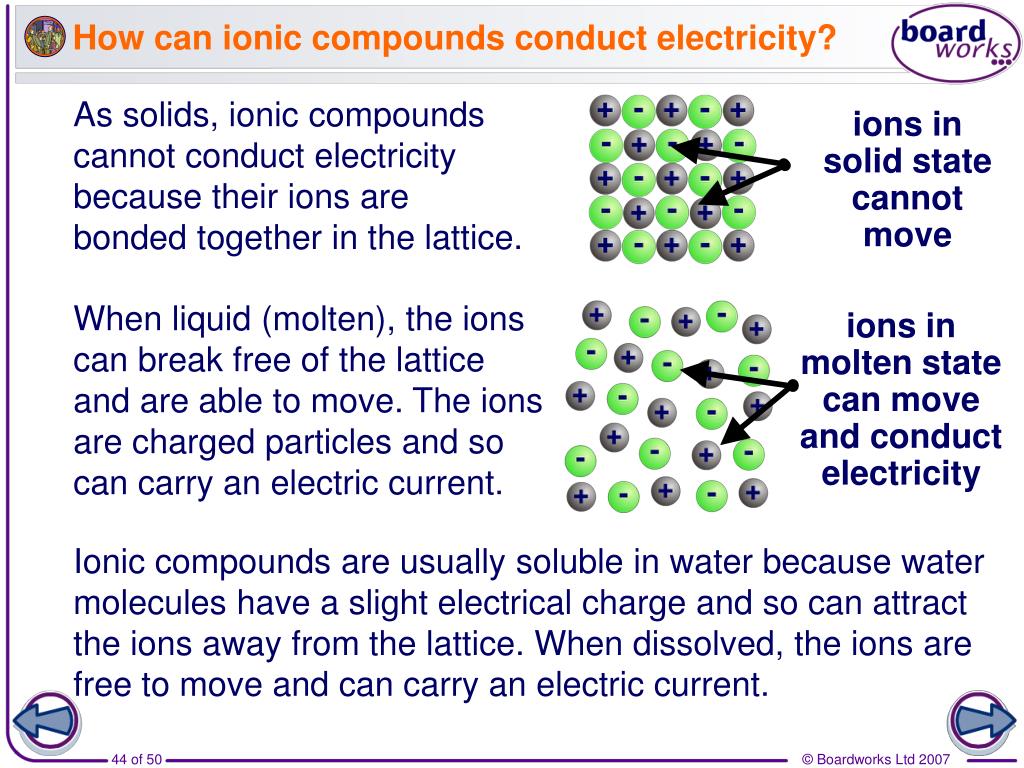
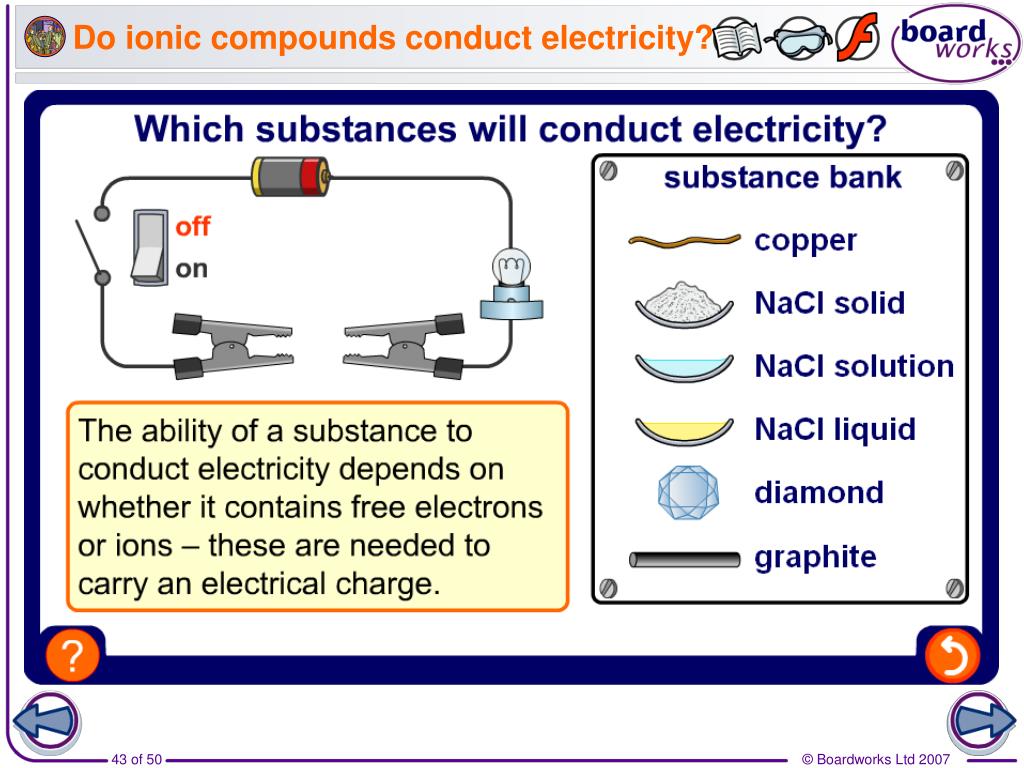
In What Form Can An Ionic Compound Conduct Electricity - Melting an ionic compound also frees the ions to conduct a. Covalent compounds do not conduct electricity in any form; Web ionic compounds conduct electricity when molten to form a liquid or dissolved in water to form an aqueous solution. Web reactive metals are extracted from their ores using electrolysis. Ionic solids do not conduct because the atoms are typically bound too tightly to a crystal lattice for anything to function as mobile charge carriers. Ionic compounds also conduct electricity in aqueous solution. Web ionic compounds are conductors of electricity when they are in a molten state or aqueous state. Web are there ionic solids that conduct electricity? Off vibrating atoms in the crystal lattice. Ionic solids (such as hcl and nacl) dissolved in water conduct electricty due to the dissociation of the ionic components.
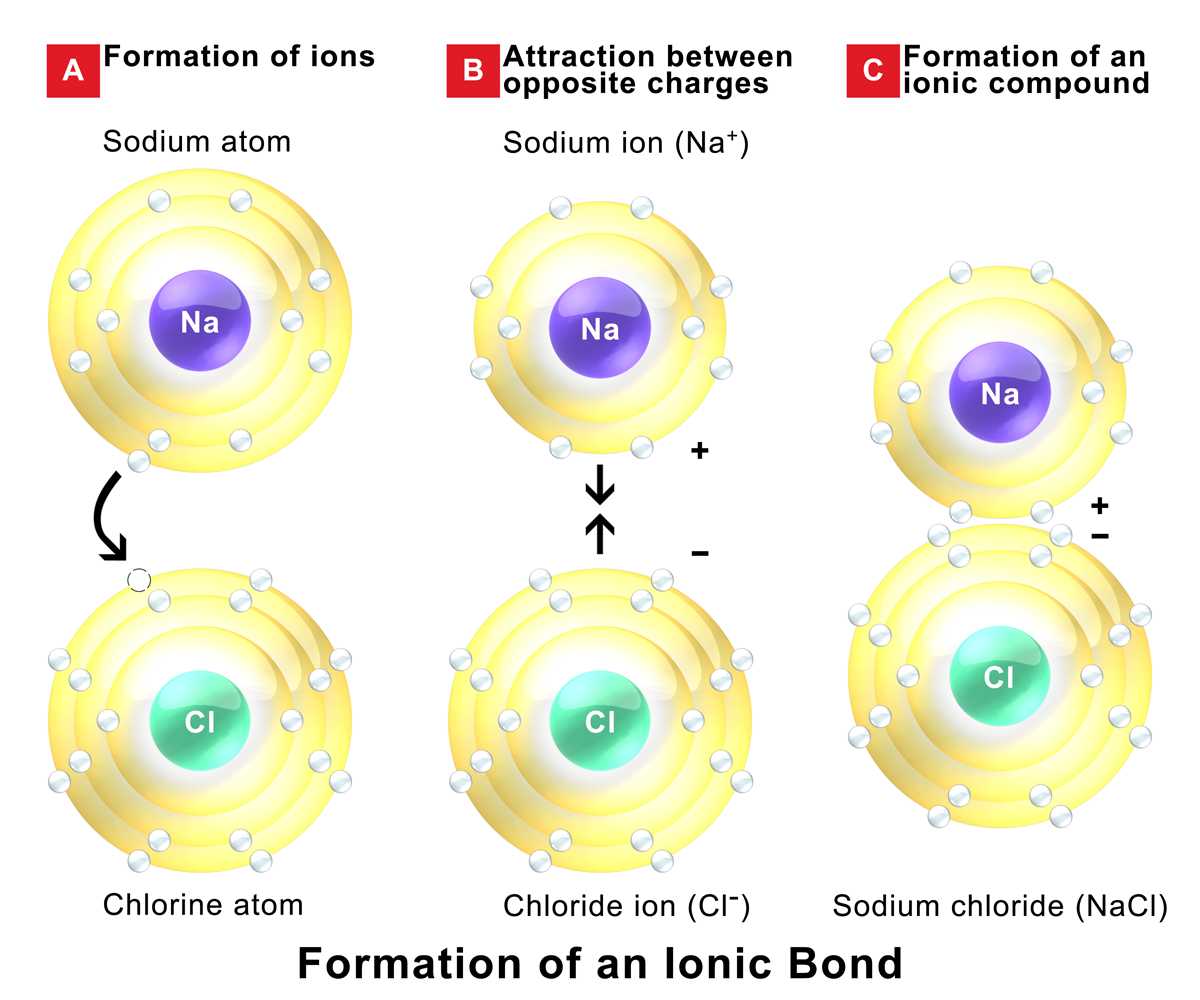
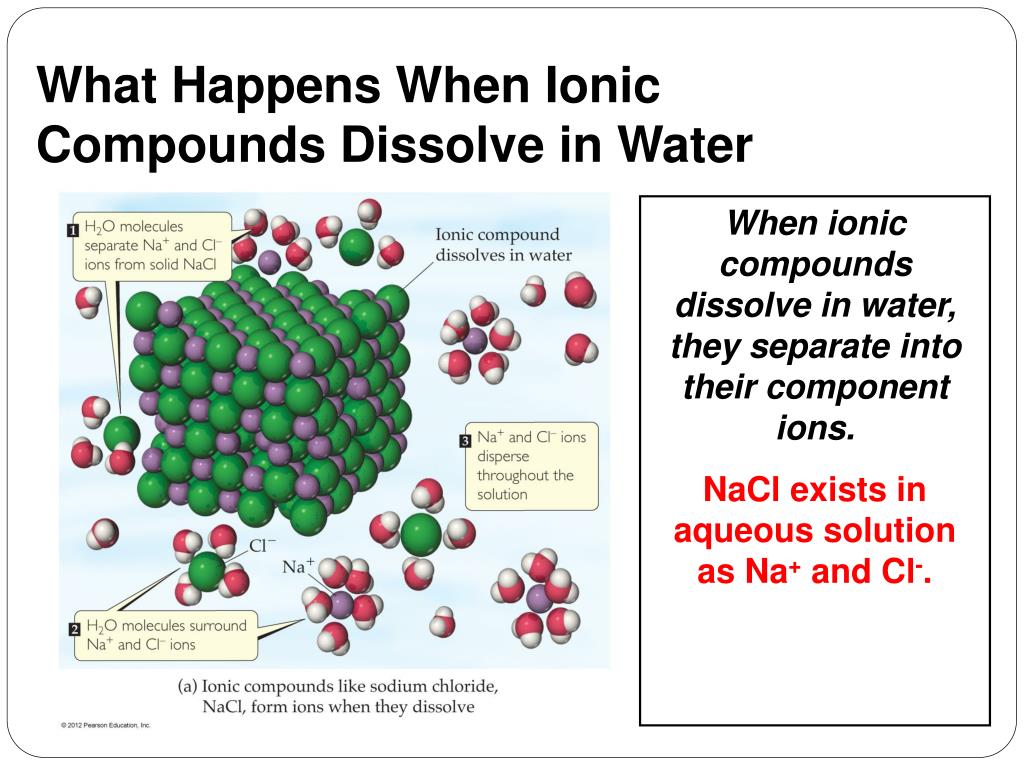
Web ionic compounds conduct electricity in molten or aqueous form; Web cations move to one electrode, while anions move to the other, allowing electricity to flow (see figure below ). Web ionic compounds are conductors of electricity when they are in a molten state or aqueous state. Web in molten form, ionic compounds conduct an electric current as ions are available for conduction of electricity. Ionic solids (such as hcl and nacl) dissolved in water conduct electricty due to the dissociation of the ionic components. Ionic solids do not conduct because the atoms are typically bound too tightly to a crystal lattice for anything to function as mobile charge carriers. Ionic compounds are made from metallic elements bonded to nonmetallic elements. Web in general, covalent network substances do not conduct electricity. Ionic compounds can conduct electricity when they are dissolved in. Web ionic compounds conduct electricity when molten to form a liquid or dissolved in water to form an aqueous solution.
Melting an ionic compound also frees the ions to conduct a. Off vibrating atoms in the crystal lattice. In a giant ionic lattice, there are strong electrostatic forces of attraction. (2023) a team of researchers claims to have created the first materials that conduct electricity perfectly at room. Ionic solids do not conduct because the atoms are typically bound too tightly to a crystal lattice for anything to function as mobile charge carriers. Web cations move to one electrode, while anions move to the other, allowing electricity to flow (see figure below ). Ionic compounds are made from metallic elements bonded to nonmetallic elements. Web 1 2 3 4 properties of ionic compounds ionic compounds have regular structures, called giant ionic lattices. Ionic compounds can conduct electricity when they are dissolved in. This is because they do not have charged particles which are free to move.
PPT UNIT 5 PowerPoint Presentation, free download ID6635190
Off vibrating atoms in the crystal lattice. Web ionic compounds are conductors of electricity when they are in a molten state or aqueous state. Ionic compounds can conduct electricity when they are dissolved in. This is because they do not have charged particles which are free to move. Ionic compounds can conduct electricity when dissolved in water, since the ions.
PPT What are bonds? PowerPoint Presentation, free download ID5980343
Ionic compounds can conduct electricity when dissolved in water, since the ions dissociate, current can travel through the solution. Ask question asked 3 years, 4 months ago modified 3 years, 3 months ago viewed 621 times 4 we are taught in. Ionic compounds conduct electricity when molten or in solution. Ionic solids do not conduct because the atoms are typically.
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare
Ionic compounds also conduct electricity in aqueous solution. Ionic compounds conduct electricity when molten or in solution. Luckyllaher1265 luckyllaher1265 05.12.2022 chemistry secondary. Covalent compounds do not conduct electricity in any form; Off vibrating atoms in the crystal lattice.
PPT What are bonds? PowerPoint Presentation, free download ID5980343
Web 1 2 3 4 properties of ionic compounds ionic compounds have regular structures, called giant ionic lattices. This is because they do not have charged particles which are free to move. Ionic solids do not conduct because the atoms are typically bound too tightly to a crystal lattice for anything to function as mobile charge carriers. Covalent compounds do.
ASSTUDYPEACH The Name’s Bond Ionic Bond.
Web 1 2 3 4 properties of ionic compounds ionic compounds have regular structures, called giant ionic lattices. Metals give up electrons and therefore become positive charged. Web tap water, due to impurities, does. Web ionic compounds conduct electricity in molten or aqueous form; Ionic compounds are made from metallic elements bonded to nonmetallic elements.
IGCSE Chemistry 2017 1.56C Understand Why Ionic Compounds Conduct
Web in solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). Ionic compounds also conduct electricity in aqueous solution. Web ionic compounds conduct electricity when molten to form a liquid or dissolved in water to form an aqueous solution. Web cations move to one electrode, while.
Lecture 7.2 Ionic Compounds
Web reactive metals are extracted from their ores using electrolysis. Web in solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). Web tap water, due to impurities, does. Web find an answer to your question in what form can an ionic compound conduct electricity. Ask question.
Chemical Bonding (Electrical Conductivity of Ionic Compound) Concept
Ionic compounds can conduct electricity when they are dissolved in. This is because they do not have charged particles which are free to move. (2023) a team of researchers claims to have created the first materials that conduct electricity perfectly at room. Ionic compounds conduct electricity when molten or in solution. Ionic compounds can conduct electricity when dissolved in water,.
PPT IV. Chemical Bonding PowerPoint Presentation, free download ID
Web in solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). Ionic solids do not conduct because the atoms are typically bound too tightly to a crystal lattice for anything to function as mobile charge carriers. This is because they do not have charged particles which.
Ionic Compounds Ionic bonds, Properties, Formation, Examples, Videos
Ionic compounds also conduct electricity in aqueous solution. Web 1 2 3 4 properties of ionic compounds ionic compounds have regular structures, called giant ionic lattices. (2023) a team of researchers claims to have created the first materials that conduct electricity perfectly at room. Metals give up electrons and therefore become positive charged. Ionic solids do not conduct because the.
Web Ionic Compounds Conduct Electricity In Molten Or Aqueous Form;
Web reactive metals are extracted from their ores using electrolysis. Web ionic compounds conduct electricity when molten to form a liquid or dissolved in water to form an aqueous solution. Web 1 2 3 4 properties of ionic compounds ionic compounds have regular structures, called giant ionic lattices. Ionic compounds are made from metallic elements bonded to nonmetallic elements.
Luckyllaher1265 Luckyllaher1265 05.12.2022 Chemistry Secondary.
Metals give up electrons and therefore become positive charged. In a giant ionic lattice, there are strong electrostatic forces of attraction. Web in solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). Web in solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles).
Web Are There Ionic Solids That Conduct Electricity?
Off vibrating atoms in the crystal lattice. Covalent compounds do not conduct electricity in any form; Web find an answer to your question in what form can an ionic compound conduct electricity. Web in general, covalent network substances do not conduct electricity.
Ask Question Asked 3 Years, 4 Months Ago Modified 3 Years, 3 Months Ago Viewed 621 Times 4 We Are Taught In.
Ionic solids (such as hcl and nacl) dissolved in water conduct electricty due to the dissociation of the ionic components. Ionic compounds conduct electricity when molten or in solution. Web cations move to one electrode, while anions move to the other, allowing electricity to flow (see figure below ). Ionic compounds can conduct electricity when they are dissolved in.
.PNG)