What Is Crf Form
What Is Crf Form - Crf as abbreviation means certificate request form. Web conditional random fields ( crfs) are a class of statistical modeling methods often applied in pattern recognition and machine learning and used for structured prediction. You define the form in an excel spreadsheet file for use. Crf is a kind of kidney failure at a gradual pace. Case report form (crf) is a specialized document in clinical research. Web the full form of crf is chronic renal failure. Web what is an electronic case report form (ecrf)? The crf is used by the study sponsor to capture and retain important data in the. Web a case report form (crf) is an electronic or paper document which is used in a clinical trial to record the protocol and required information about each. Web one of the definitions of crf is certificate request form.
Web the daids rsc case report form (crf) management team works closely with daids and clinical research sites to facilitate this process. Case report form (crf) is a specialized document in clinical research. Container request form * crf: This page is designed to walk users. You define the form in an excel spreadsheet file for use. Crf is a kind of kidney failure at a gradual pace. Web this set of topics explains how you define (create) case report forms (crfs) and make changes to the defined forms. Web the full form of crf is chronic renal failure. Clean report of findings (used in shipments and customs environment) crf: Contingency response force ** crf:
Crf as abbreviation means certificate request form. Web a crf is a set of documents that collects data and information from a clinical trial. It should be study protocol driven, robust in content and have material to collect the study specific. You define the form in an excel spreadsheet file for use. The crf is used by the study sponsor to capture and retain important data in the. Web according to ich gcp ec 1.11, a case report form is a printed, optical, or electronic document designed to record all of the protocol required information to be. Web the full form of crf is chronic renal failure. Case report form (crf) is a specialized document in clinical research. Contingency response force ** crf: Community regeneration fund (scottish executive, scotland).
Form Crf002 State Tax Registration Application printable pdf download
Web what is an electronic case report form (ecrf)? Web one of the definitions of crf is certificate request form. Crf as abbreviation means certificate request form. It should be study protocol driven, robust in content and have material to collect the study specific. Moreover, renal insufficiency is at its maximum level in this kind of.
CRF Full Form In Hindi CRF Se Kya Samjhte Hai PagalNews
Web what is an electronic case report form (ecrf)? You define the form in an excel spreadsheet file for use. Web a crf is a set of documents that collects data and information from a clinical trial. Moreover, renal insufficiency is at its maximum level in this kind of. Cryptographic repair facility * crf:
Form Crf014 CoinOperated Amusement Machines Renewal Application
Container request form * crf: The crf is used by the study sponsor to capture and retain important data in the. Crf as abbreviation means certificate request form. Cryptographic repair facility * crf: Contingency response force ** crf:
Fillable Form Crf002 State Tax Registration Application printable
Case report form (crf) is a specialized document in clinical research. The crf is used by the study sponsor to capture and retain important data in the. Web what is an electronic case report form (ecrf)? Crf is a kind of kidney failure at a gradual pace. Web according to ich gcp ec 1.11, a case report form is a.
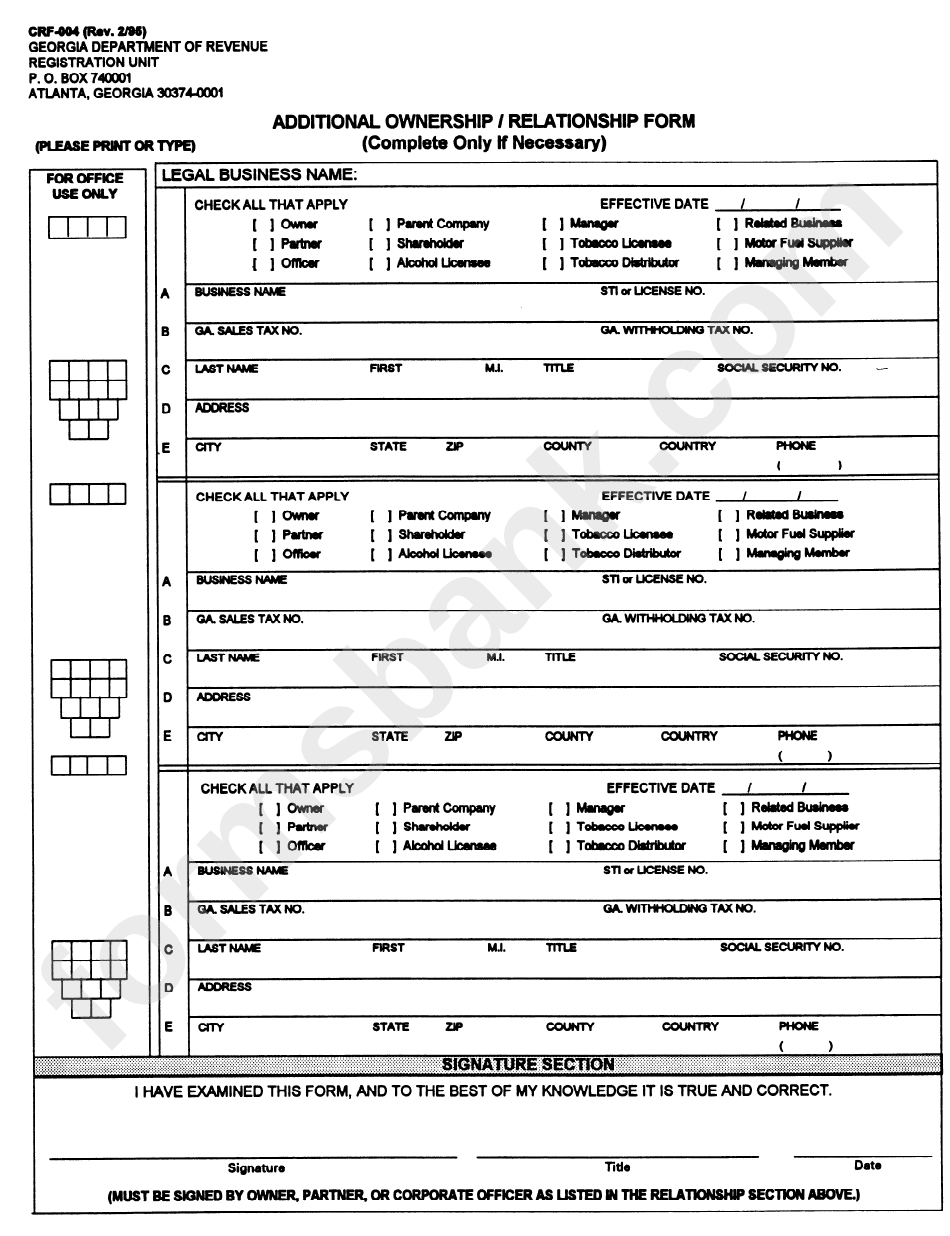
Form Crf004 Additional Ownership / Relationship Form printable pdf
Web what is an electronic case report form (ecrf)? Web the full form of crf is chronic renal failure. Crf as abbreviation means certificate request form. Cryptographic repair facility * crf: Community regeneration fund (scottish executive, scotland).
Form Crf002 State Tax Registration Application printable pdf download
Web a case report form (crf) is an electronic or paper document which is used in a clinical trial to record the protocol and required information about each. Community regeneration fund (scottish executive, scotland). Web what is an electronic case report form (ecrf)? Web one of the definitions of crf is certificate request form. You define the form in an.
Longterm followup form. Simple one page CRF for collection of primary
Web this set of topics explains how you define (create) case report forms (crfs) and make changes to the defined forms. Web one of the definitions of crf is certificate request form. Web a case report form (crf) is an electronic or paper document which is used in a clinical trial to record the protocol and required information about each..
Form Crf004 Additional Ownership / Relationship Form printable pdf
Web this questionnaire is known as a case report form, or crf, and it contains participant data so researchers can categorize and analyze it during the next phase of. Web a crf is a set of documents that collects data and information from a clinical trial. Web the daids rsc case report form (crf) management team works closely with daids.
Form Crf008 Tobacco License Application printable pdf download
You define the form in an excel spreadsheet file for use. Web the full form of crf is chronic renal failure. This page is designed to walk users. Clean report of findings (used in shipments and customs environment) crf: Community regeneration fund (scottish executive, scotland).
Tutor FAQs for Academic Success Center
This page is designed to walk users. Web this set of topics explains how you define (create) case report forms (crfs) and make changes to the defined forms. You define the form in an excel spreadsheet file for use. Web a case report form (crf) is an electronic or paper document which is used in a clinical trial to record.
Web Conditional Random Fields ( Crfs) Are A Class Of Statistical Modeling Methods Often Applied In Pattern Recognition And Machine Learning And Used For Structured Prediction.
Crf is a kind of kidney failure at a gradual pace. Web a crf is a set of documents that collects data and information from a clinical trial. Web one of the definitions of crf is certificate request form. Web the full form of crf is chronic renal failure.
Cryptographic Repair Facility * Crf:
Web the daids rsc case report form (crf) management team works closely with daids and clinical research sites to facilitate this process. Community regeneration fund (scottish executive, scotland). Web according to ich gcp ec 1.11, a case report form is a printed, optical, or electronic document designed to record all of the protocol required information to be. Crf as abbreviation means certificate request form.
This Page Is Designed To Walk Users.
Case report form (crf) is a specialized document in clinical research. Clean report of findings (used in shipments and customs environment) crf: Container request form * crf: Web what is an electronic case report form (ecrf)?
Construction Reserve Fund ** Crf:
The crf is used by the study sponsor to capture and retain important data in the. It should be study protocol driven, robust in content and have material to collect the study specific. Web a case report form (crf) is an electronic or paper document which is used in a clinical trial to record the protocol and required information about each. Web this questionnaire is known as a case report form, or crf, and it contains participant data so researchers can categorize and analyze it during the next phase of.