Will Argon Tend To Form Bonds With Other Elements
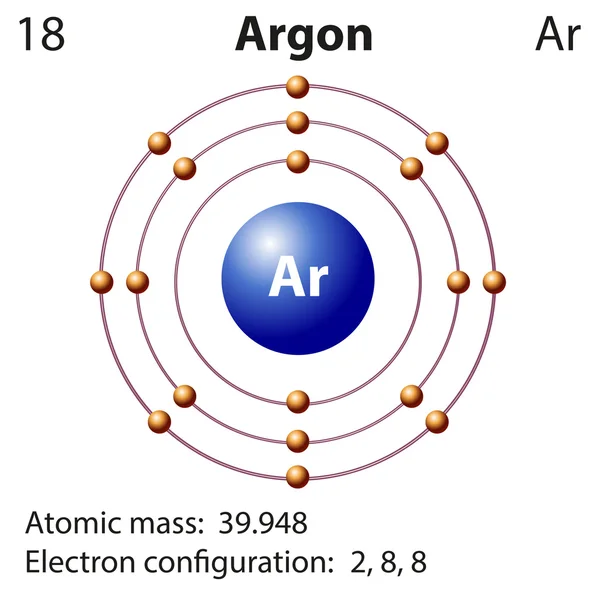
Will Argon Tend To Form Bonds With Other Elements - The atomic number of argon is 18. Will argon tend to form bonds with other elements? But in the 23 august issue of nature, chemists report that. A full valence shell is the most stable electron configuration. Web group 18 elements (helium, neon, and argon) have a full outer, or valence, shell. Will argon tend to form bonds with other elements? Web it does not form bonds with other elements. A clue comes by considering the noble gas elements, the. No, it generally does not form chemical bonds because it is a noble. The maximum number of electrons that can be held in the orbitals in an atom’s second energy level is 2.
Web note the usefulness of the periodic table in predicting likely ion formation and charge (figure \(\pageindex{2}\)). Please check the table and with the element argon listed, because how can it give/get/share if it. Web what causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms? Unhappy elements formed bonds to create molecules, whereas happy elements remained. Web the element argon has always been a loner. Group 1 element atoms form a metallically bonded lattice. Web the number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms. But in the 23 august issue of nature, chemists report that. Will argon tend to form bonds with other elements? The maximum number of electrons that can be held in the orbitals in an atom’s second energy level is 2.
Will argon tend to form bonds with other elements? A full valence shell is the most stable electron configuration. Web group 18 elements (helium, neon, and argon) have a full outer, or valence, shell. It's one of the inert gases that normally exist as single atoms. Argon atoms would not tend to make bonds with other atoms since it is classified as a noble atom which means it has a complete filled subshells. Will argon tend to form bonds with other elements? Elements follow what is commonly termed the 8 is great. Web it does not form bonds with other elements. Group 1 element atoms form a metallically bonded lattice. Therefore, based on its electron configuration and position on the.
Why do Noble Gases rarely form Bonds with other Atoms? MakeTheBrainHappy
Web the number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms. Elements in other groups have. Therefore, based on its electron configuration and position on the. Will argon tend to form bonds with other elements? Elements follow what is commonly termed the 8 is great.
Argon form Periodic Table of Elements — Stock Photo © fambros 3096120
No, it generally does not form chemical bonds because it is a noble. Web biology questions and answers. Argon atoms would not tend to make bonds with other atoms since it is classified as a noble atom which means it has a complete filled subshells. The atomic number of argon is 18. Moving from the far left to the right.
Reactivity CLASSWORK Why are argon and neon unlikely to... Math
Web group 18 elements (helium, neon, and argon) have a full outer, or valence, shell. Web elements usually end up forming a giant metallically bonded lattice if they have a low number of valence electrons. Argon atoms would not tend to make bonds with other atoms since it is classified as a noble atom which means it has a complete.
Sample from the RGB Set, a sample of the element Argon in the Periodic
Moving from the far left to the right on the periodic. Web if argon doesn’t form ions to bond with other elements can it even be ionic? No, it generally does not form chemical bonds because it is a noble. Unhappy elements formed bonds to create molecules, whereas happy elements remained. A clue comes by considering the noble gas elements,.
Diagram representation of the element neon Stock Vector Image by
Please check the table and with the element argon listed, because how can it give/get/share if it. Web the atomic number of argon is 18. Argon is one of the noble gases (group 18) and thus does not normally combine with other elements. Web if argon doesn’t form ions to bond with other elements can it even be ionic? Web.
1 of 18 ng Goal Review Constants I Per... Physical Chemistry
Will argon tend to form bonds with other elements? The atomic number of argon is 18. Web the element argon has always been a loner. A full valence shell is the most stable electron configuration. Moving from the far left to the right on the periodic.
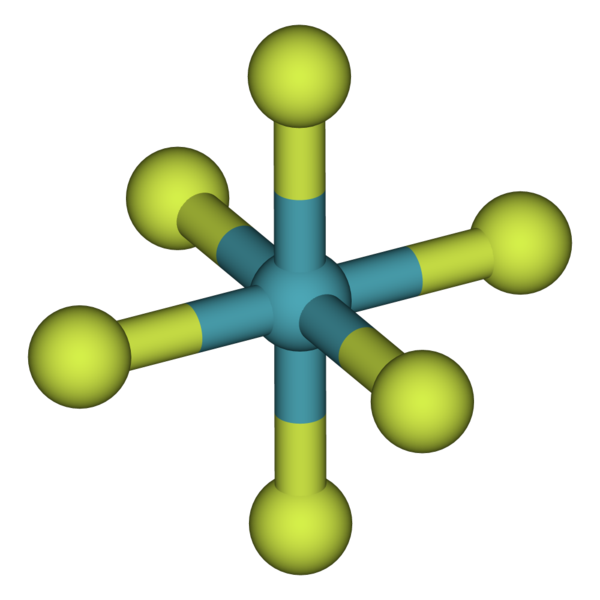
Argon atoms form cylindrical configurations after collision of two
Therefore, based on its electron configuration and position on the. A full valence shell is the most stable electron configuration. Web the number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms. Will argon tend to form bonds with other elements? Web group 18 elements (helium, neon,.
Reading Covalent Bonds Biology I
Elements follow what is commonly termed the 8 is great. Argon atoms would not tend to make bonds with other atoms since it is classified as a noble atom which means it has a complete filled subshells. Web it does not form bonds with other elements. Web biology questions and answers. Moving from the far left to the right on.
Argon Form Periodic Table Of Elements Stock Illustration Illustration
Web if argon doesn’t form ions to bond with other elements can it even be ionic? Web the atomic number of argon is 18. Web it does not form bonds with other elements. This outermost shell is known. Web group 18 elements (helium, neon, and argon) have a full outer, or valence, shell.
The Atomic Number Of Argon Is 18.
No, it generally does not form chemical bonds because it is a noble. It's one of the inert gases that normally exist as single atoms. Will argon be likely to form bonds with other elements? Elements follow what is commonly termed the 8 is great.
Web What Causes Atoms To Make A Chemical Bond With Other Atoms, Rather Than Remaining As Individual Atoms?
Elements in other groups have. But in the 23 august issue of nature, chemists report that. Please check the table and with the element argon listed, because how can it give/get/share if it. Therefore, based on its electron configuration and position on the.
Web The Element Argon Has Always Been A Loner.
Unhappy elements formed bonds to create molecules, whereas happy elements remained. Web argon's name comes from the greek word argos meaning lazy and indeed for more than a hundred years after its discovery chemists were unable to get it to combine with any. Web it does not form bonds with other elements. Group 1 element atoms form a metallically bonded lattice.
Web Elements Usually End Up Forming A Giant Metallically Bonded Lattice If They Have A Low Number Of Valence Electrons.
This outermost shell is known. Web the atomic number of argon is 18. Web this makes it a noble gas, which are generally unreactive and do not tend to form bonds with other elements. Argon is one of the noble gases (group 18) and thus does not normally combine with other elements.