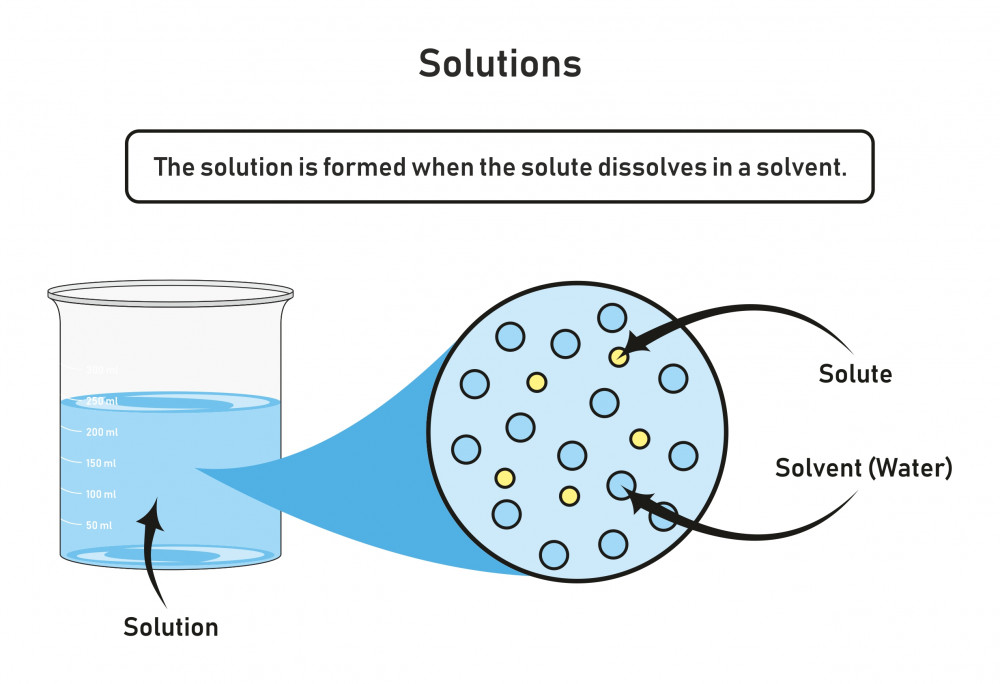
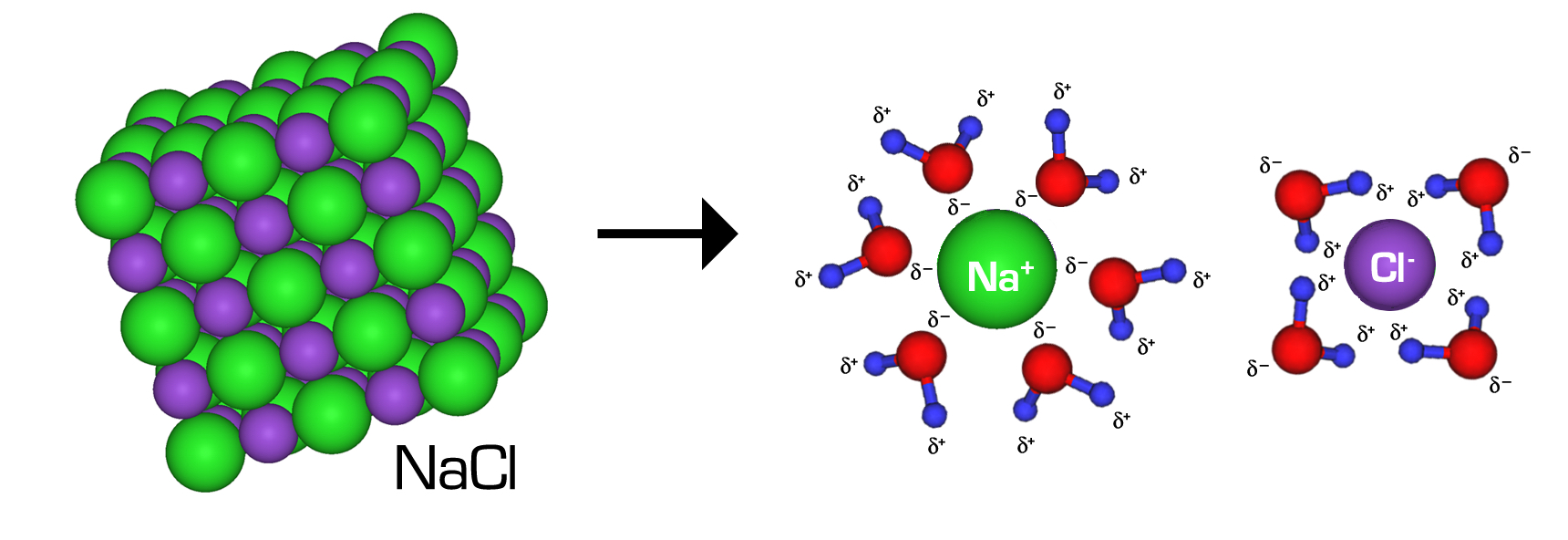
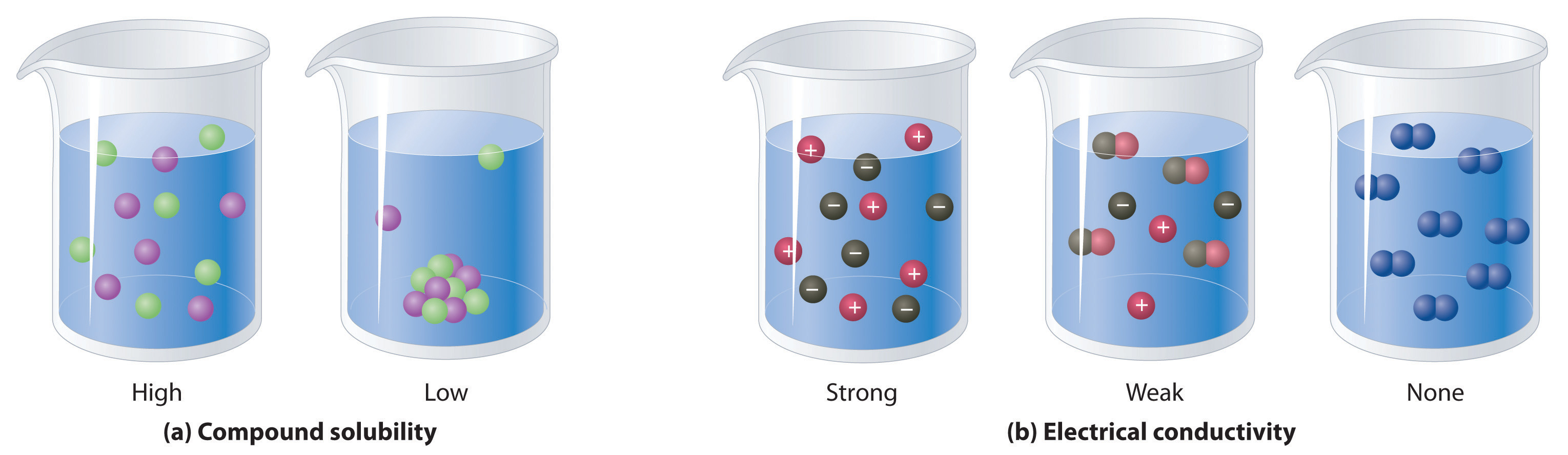
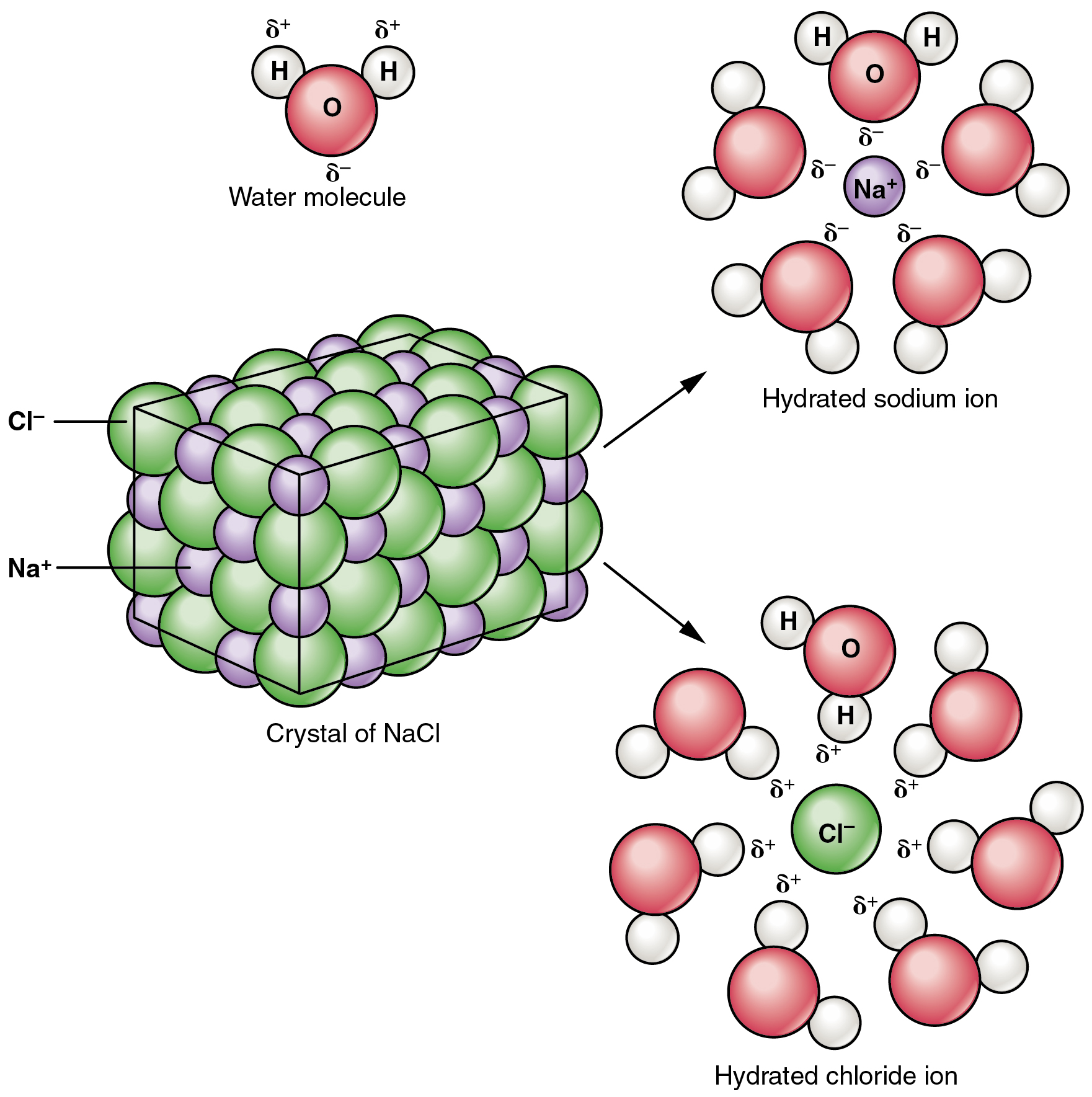
A Dissolved Solute That Does Not Form Ions Is
A Dissolved Solute That Does Not Form Ions Is - Web solutes that dissolve into individual neutral molecules without dissociation do not impart additional electrical conductivity to their solutions and are called. Web when dissolution happens, the solute separates into ions or molecules, and each ion or molecule is surrounded by molecules of solvent. Web a solute that does not dissociate into ions in aqueous solution. Typically, this involves a solid. Web the components of a solution are dispersed on a molecular scale; Web dissolution is the process where a solute in gaseous, liquid, or solid phase dissolves in a solvent to form a solution. To bring to an end : Web the dissolved solute in a solution will not settle out or separate from the solvent. Dissolving is also called dissolution. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water.
Web insolubility is the opposite property, the inability of the solute to form such a solution. Web solute or aqueous solution that does not conduct electricity. The dissolved solute in a solution. Web updated on may 07, 2019. To bring to an end : Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Solubility at the maximum concentration of. To separate into component parts : Web an electrolyte refers to a substance that contains free ions and can be used as an electrically conductive medium. Web a solute that does not dissociate into ions in aqueous solution.
Solubility at the maximum concentration of. The vapor pressure of the. The dissolved solute in a solution. Typically, this involves a solid. The dissolved solute in a solution. Web the components of a solution are dispersed on a molecular scale; Web an electrolyte refers to a substance that contains free ions and can be used as an electrically conductive medium. Most of the solute does not dissociate in a weak. Which does not affect the rate at which a solid solute dissolves. Which type of mixture contains the smallest particles.
CH150 Chapter 7 Solutions Chemistry
The dissolved solute in a solution. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. [verb] to cause to disperse or disappear : Web solutes that dissolve into individual neutral molecules without dissociation do not impart additional electrical conductivity to their solutions and are called. Web the components of a solution are dispersed on.
Chemistry Solutions And Mixtures Level 2 activity for kids
Web solute or aqueous solution that does not conduct electricity. Web solutes that dissolve into individual neutral molecules without dissociation do not impart additional electrical conductivity to their solutions and are called. Web when a small amount of solid potassium dichromate is added to water, the compound dissolves and dissociates to yield potassium ions and dichromate ions uniformly. Web the.
Do Polar Or Nonpolar Molecules Dissolve In Water Drawhub
Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Web the components of a solution are dispersed on a molecular scale; That is, they consist of a mixture of separated molecules, atoms, and/or ions. Web dissolution is the process where a solute in gaseous, liquid, or solid phase dissolves in a solvent to form.
AP Chemistry Unit 3 Solution Chemistry PRE POST Quiz Quizizz
In chemistry, to dissolve is to cause a solute to pass into a solution. To bring to an end : [verb] to cause to disperse or disappear : Web a solute that does not dissociate into ions in aqueous solution. Web the process of surrounding solute particles with solvent particles to form a solution is called _____.
The image shows particles of salt dissolved in water. Brainly.in
The vapor pressure of the. That is, they consist of a mixture of separated molecules, atoms, and/or ions. Most of the solute does not dissociate in a weak. Web a solute that does not dissociate into ions in aqueous solution. Web an electrolyte refers to a substance that contains free ions and can be used as an electrically conductive medium.
Introduction to Solutions CK12 Foundation
That is, they consist of a mixture of separated molecules, atoms, and/or ions. Solubility at the maximum concentration of. To separate into component parts : That is, they consist of a mixture of separated molecules, atoms, and/or ions. Physical state in which the opposing processes of dissolution and crystallization of a solute occur.
4.1 General Properties of Aqueous Solutions Chemistry LibreTexts
That is, they consist of a mixture of separated molecules, atoms, and/or ions. Web insolubility is the opposite property, the inability of the solute to form such a solution. Solvation if the solubility of a particular solute is 10g/100g h2o at 20c,. Web updated on may 07, 2019. Which type of mixture contains the smallest particles.
Solved How does a dissolved nonvolatile solute affect the
Web a solute that does not dissociate into ions in aqueous solution. Web the components of a solution are dispersed on a molecular scale; Web solute or aqueous solution that does not conduct electricity. Web when dissolution happens, the solute separates into ions or molecules, and each ion or molecule is surrounded by molecules of solvent. Web insolubility is the.
This figure shows a crystal lattice of sodium chloride interacting with
Web insolubility is the opposite property, the inability of the solute to form such a solution. To bring to an end : The extent of the solubility of a substance in a specific solvent is generally measured as the. Solubility at the maximum concentration of. The dissolved solute in a solution.
science chemistry experiment solubility Fundamental Photographs The
Solvation if the solubility of a particular solute is 10g/100g h2o at 20c,. Web an electrolyte refers to a substance that contains free ions and can be used as an electrically conductive medium. Web dissolution is the process where a solute in gaseous, liquid, or solid phase dissolves in a solvent to form a solution. Web if the solvent is.
Which Does Not Affect The Rate At Which A Solid Solute Dissolves.
Web a solute that does not dissociate into ions in aqueous solution. Web water typically dissolves most ionic compounds and polar molecules. Web the dissolved solute in a solution will not settle out or separate from the solvent. Web the components of a solution are dispersed on a molecular scale;
Web The Process Of Surrounding Solute Particles With Solvent Particles To Form A Solution Is Called _____.
Web if the solvent is water, hydration occurs when the charged solute ions become surrounded by water molecules. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. To separate into component parts : Web when a small amount of solid potassium dichromate is added to water, the compound dissolves and dissociates to yield potassium ions and dichromate ions uniformly.
To Bring To An End :
The dissolved solute in a solution. The dissolved solute in a solution. Physical state in which the opposing processes of dissolution and crystallization of a solute occur. Web updated on may 07, 2019.
Web The Components Of A Solution Are Dispersed On A Molecular Scale;
That is, they consist of a mixture of separated molecules, atoms, and/or ions. Web solutes that dissolve into individual neutral molecules without dissociation do not impart additional electrical conductivity to their solutions and are called. Dissolving is also called dissolution. [verb] to cause to disperse or disappear :